Breast cancer pathophysiology: Difference between revisions
No edit summary |
No edit summary |
||
| Line 94: | Line 94: | ||
# Inherited defects in [[DNA repair genes]], such as ''BRCA1'', ''BRCA2'' and ''p53''. | # Inherited defects in [[DNA repair genes]], such as ''BRCA1'', ''BRCA2'' and ''p53''. | ||
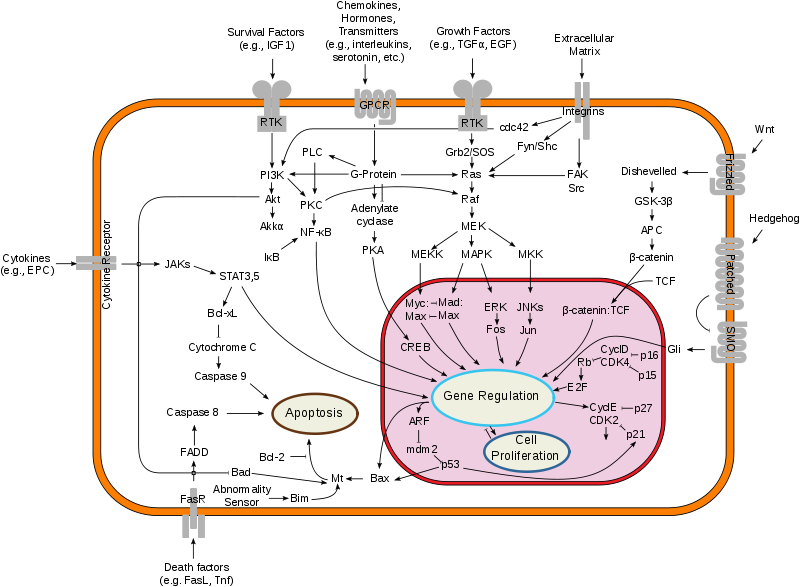
[[Image:Breast cancer.png|thumb|left| | [[Image:Breast cancer.png|thumb|left|600px|Overview of signal transduction pathways involved in programmed cell death. Mutations leading to loss of this ability can lead to cancer formation.https://en.wikipedia.org/wiki/Breast_cancer.]] | ||
===Bone Metastasis=== | ===Bone Metastasis=== | ||
Revision as of 14:57, 22 March 2019
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1] Associate Editor(s)-in-Chief: Soroush Seifirad, M.D.[2]
|
Breast Cancer Microchapters |
|
Diagnosis |
|---|
|
Treatment |
|
Case Studies |
|
Breast cancer pathophysiology On the Web |
|
American Roentgen Ray Society Images of Breast cancer pathophysiology |
|
Risk calculators and risk factors for Breast cancer pathophysiology |
Overview
Genes involved in the pathogenesis of breast cancer include BRCA1, BRCA2 and p53. On microscopic histopathological analysis, minimal tubule formation, marked pleomorphism, and numerous mitotic figures are characteristic findings of breast cancer.
Pathogenesis
- Majority of breast malignancies arise from epithelial cells and hence are carcinomas.
- Microscopic appearance and biologic behavior divide breast carcinomas to several diverse groups.
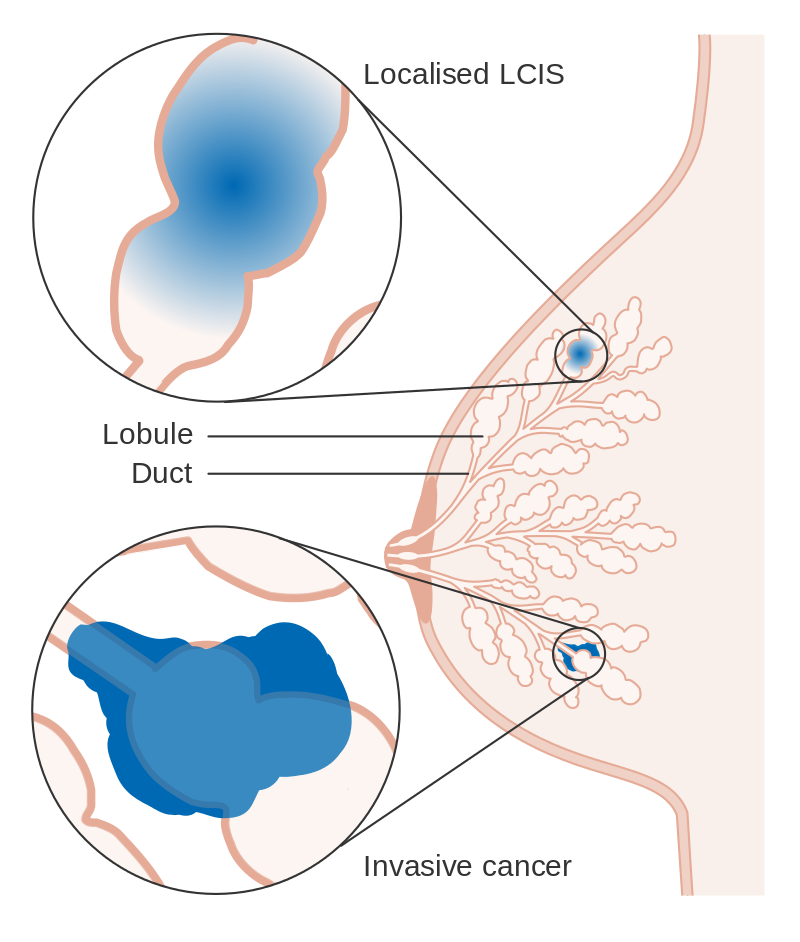
- Based upon the growth pattern and cytologic features of the lesions,the in situ carcinomas of the breast divide into ductal (also known as intraductal carcinoma) or lobular.
- Surprisingly their anatomic location within the mammary ductal-lobular system.
- The table below shows the invasive breast carcinomas several histologic subtypes; based on a series of 135,157 women with breast cancer reported to the SEER database o between 1992 and 2001.
| Histologic subtypes of invasive breast carcinomas | |
|---|---|
| Infiltrating ductal | 76 % |
| Invasive lobular | 8 % |
| Ductal/lobular | 7 % |
| Mucinous (colloid) | 2.4 % |
| Tubular | 1.5 % |
| Medullary | 1.2 % |
| Papillary | 1 % |
| Other subtypes, including:
● Metaplastic breast cancer ● Invasive micropapillary breast cancer |
less than 5 % |
- Ductal carcinoma in situ (DCIS) is described as a proliferation of abnormal cells confined within the mammary ductal system.
- DCIS is commonly classified with respect to architectural and cytologic features as well as cell necrosis as follows:
- Low and intermediate grade (papillary, cribriform, and solid)
- High grade (comedo).
- DCIS signifies a herald to invasive breast cancer.
- The invasive breast carcinomas comprise numerous histologic subtypes.
- Infiltrating ductal carcinoma (the most common)
- Infiltrating lobular carcinoma (the second most common)
- Compared to the infiltrating ductal carcinomas, infiltrating lobular carcinomas are:
- Generally multicentric or may be bilateral
- Usually more differentiated
- Hormone receptor-positive
- May arise in older women
- As a role they metastasize later but if metastasize,
- Sometimes spread to unusual locations, such as:
- The meninges
- The peritoneum
- The gastrointestinal tract
Microscopic Pathology
- Shown below is a micrograph of high grade invasive ductal carcinoma showing minimal tubule formation,marked pleomorphism, and numerous mitotic figures.



Genetics
- Breast cancer, like other cancers, occurs because of an interaction between the environment and a defective gene. Normal cells divide as many times as needed and stop; they attach to other cells and stay in place in tissues. Cells become cancerous when mutations destroy their ability to stop dividing, to attach to other cells, and to stay where they belong.
- Normal cells will commit cell suicide (apoptosis) when they are no longer needed. Until then, they are protected from cell suicide by several protein clusters and pathways. One of the protective pathways is the PI3K/AKT pathway; another one is the RAS/MEK/ERK pathway. Sometimes the genes along these protective pathways are mutated in a way that turns them permanently "on", rendering the cell incapable of committing suicide when it is no longer needed. This is one of the steps that causes cancer in combination with other mutations. Normally, the PTEN protein turns off the PI3K/AKT pathway when the cell is ready for cell suicide. In some breast cancers, the gene for the PTEN protein is mutated, so the PI3K/AKT pathway is stuck in the "on" position, and the cancer cell does not commit suicide.[1]
- Mutations that can lead to breast cancer have been experimentally linked to estrogen exposure[2] and failure of immune surveillance, the removal of malignant cells throughout one's life by the immune system.[3]
- Abnormal growth factor signaling in the interaction between stromal cells and epithelial cells can facilitate malignant cell growth.[4][5] In breast adipose tissue, overexpression of leptin leads to increased cell proliferation and cancer.[6]
- In the United States, 10 to 20 % of patients with breast cancer or ovarian cancer have a first- or second-degree relative with one of these diseases. The familial tendency to develop these cancers is called hereditary breast—ovarian cancer syndrome. The best known of these, the BRCA mutations, confer a lifetime risk of breast cancer of between 60 and 85 % and a lifetime risk of ovarian cancer of between 15 and 40 %. Some mutations associated with cancer, such as p53, BRCA1 and BRCA2, occur in mechanisms to correct errors in DNA. These mutations are either inherited or acquired after birth. Presumably, they allow further mutations, which lead to uncontrolled division, lack of attachment, and metastasis to distant organs.[7][8]However, there is strong evidence of residual risk variation that goes well beyond hereditary BRCA gene mutations between carrier families; this is caused by unobserved risk factors.[9] It implicates that environmental factors and other causes are triggers for breast cancer. The inherited mutation in BRCA1 or BRCA2 genes can interfere with repair of DNA cross links and DNA double strand breaks (known functions of the encoded protein).[10] Because of this repair deficit, risks from carcinogenic chemicals and ionizing radiation can increase.[11]These carcinogens cause DNA damage, such as to DNA cross links and double strand breaks that often require repairs by pathways containing BRCA1 and BRCA2.[12][13] But it is these repair pathways that can be crippled by inherited mutation. There is evidence that cancer risks increase in mutation carriers exposed to such opportunistic carcinogens.[14] Thus, risks for cancers may be reduced by avoiding or compensating for carcinogens that exploit the inherited BRCA gene deficiency[15] However, mutations in BRCA genes account for only 2 to 3 % of all breast cancers.[16] About half of hereditary breast–ovarian cancer syndromes involve unknown genes.
- Today, breast cancer, like other forms of cancer, is considered to be the final outcome of multiple environmental and hereditary factors. Some of the effects of environmental and hereditary factors that ultimately cause breast cancer are:
- Lesions to DNA such as genetic mutations. Exposure to estrogen has been experimentally linked to the mutations that cause breast cancer.[2] Beyond the contribution of estrogen, research has implicated viral oncogenesis and the contribution of ionizing radiation.
- Failure of immune surveillance, which usually removes malignancies at early phases of their natural history.
- Abnormal growth factor signaling in the interaction between stromal cells and epithelial cells. For example: in the angiogenesis, it is necessary to promote new blood vessel growth near new cancers.
- Inherited defects in DNA repair genes, such as BRCA1, BRCA2 and p53.
Bone Metastasis
- Bone is the most common site of breast cancer distant spread. Bone metastases due to breast cancer cause major morbidity, decrease survival and reduce quality of life of many patients.
Cancer influence on the skeleton results in two main negative consequences: pain and Skeletal-Related events (SREs), defined as any of the following:
- pathologic fracture,
- a requirement for surgical intervention and palliative radiotherapy to bone lesions,
- spinal cord compression,
- hypercalcemia of malignancy [17].
In fact, SREs constitute readily measured clinical parameters that are employed in clinics and clinical trials.
- Many disciplines should be involved in the management of breast cancer bone metastases, including medical oncology, pain and palliative care, radiation oncology, orthopedic surgery and neurosurgery. Systemic therapy delays the progression of bone metastases and provides palliation; it includes endocrine therapy, biologic agents, chemotherapy, bisphosphonate therapy and the new osteoclast inhibitors.
- A thorough knowledge of the molecular basis of bone metastasis caused by breast cancer is essential for the understanding of the therapeutic approach. In fact, The normal balance between bone resorption and deposition is significantly affected by cancer. Bone metastases due to breast cancer are mostly osteolytic lesions, though predominant osteoblastic disease can occur [18].
The breast cancer cells and the bone microenvironment interact extensively through many chemical mediators resulting in bone destruction and tumor growth. These molecular mediators (pimarily Osteopontin, CXCR4, CTGF and Interleukin-11) exert their effect on osteoclasts which in turn cause bone resorption. This osteoclast-mediated bone resorption is thought to be the product of the action of numerous molecules including:
- PTHrP (Parathyroid Hormone–related Peptide),
- Tumor Necrosis Factor α (TNF-α),
- Cytokines such as Interleukin-1, Interleukin-6, Interleukin-8, and interleukin-11
- These factors signal osteoblasts (the bone-building cells) to induce osteoclast differentiation through the RANKL (the ligand for the receptor activator of nuclearfactor-κB [RANK])- RANK signaling. When Osteoclasts lyse bone, they cause the release of growth factors such as bone morphogenetic proteins (BMPs), IGF-I and TGF-β from the bone matrix which stimulate and maintain tumor cell proliferation and induce further release of PTHrP [19].
References
- ↑ "32nd Annual CTRC-AACR San Antonio Breast Cancer Symposium" (PDF). Sunday Morning Year-End Review. Dec. 14, 2009. Unknown parameter
|coauthors=ignored (help); Check date values in:|date=(help) - ↑ 2.0 2.1 Cavalieri E, Chakravarti D, Guttenplan J; et al. (2006). "Catechol estrogen quinones as initiators of breast and other human cancers: implications for biomarkers of susceptibility and cancer prevention". Biochimica et Biophysica Acta. 1766 (1): 63–78. doi:10.1016/j.bbcan.2006.03.001. PMID 16675129. Unknown parameter
|month=ignored (help) - ↑ Farlex (2005). "Immunological Surveilliance". The Free Dictionary. Retrieved 2008-02-10.
- ↑ Haslam SZ, Woodward TL (2003). "Host microenvironment in breast cancer development: epithelial-cell-stromal-cell interactions and steroid hormone action in normal and cancerous mammary gland". Breast Cancer Res. 5 (4): 208–15. doi:10.1186/bcr615. PMC 165024. PMID 12817994. Unknown parameter
|month=ignored (help) - ↑ Wiseman BS, Werb Z (2002). "Stromal effects on mammary gland development and breast cancer". Science. 296 (5570): 1046–9. doi:10.1126/science.1067431. PMC 2788989. PMID 12004111. Unknown parameter
|month=ignored (help) - ↑ Jardé T, Perrier S, Vasson MP, Caldefie-Chézet F (2011). "Molecular mechanisms of leptin and adiponectin in breast cancer". Eur. J. Cancer. 47 (1): 33–43. doi:10.1016/j.ejca.2010.09.005. PMID 20889333. Unknown parameter
|month=ignored (help) - ↑ American Cancer Society (2005). "Breast Cancer Facts & Figures 2005–2006" (PDF). Archived from the original (PDF) on June 13, 2007. Retrieved 2007-04-26.
- ↑ Dunning AM, Healey CS, Pharoah PD, Teare MD, Ponder BA, Easton DF (1999). "A systematic review of genetic polymorphisms and breast cancer risk". Cancer Epidemiology, Biomarkers & Prevention. 8 (10): 843–54. PMID 10548311. Unknown parameter
|month=ignored (help) - ↑ Begg CB, Haile RW, Borg A; et al. (2008). "Variation of breast cancer risk among BRCA1/2 carriers". JAMA. 299 (2): 194–201. doi:10.1001/jama.2007.55-a. PMC 2714486. PMID 18182601. Unknown parameter
|month=ignored (help) - ↑ Patel KJ, Yu VP, Lee H; et al. (1998). "Involvement of Brca2 in DNA repair". Mol. Cell. 1 (3): 347–57. doi:10.1016/S1097-2765(00)80035-0. PMID 9660919. Unknown parameter
|month=ignored (help) - ↑ Friedenson B (2000). "Is mammography indicated for women with defective BRCA genes? Implications of recent scientific advances for the diagnosis, treatment, and prevention of hereditary breast cancer". MedGenMed. 2 (1): E9. PMID 11104455. Unknown parameter
|month=ignored (help) - ↑ Marietta C, Thompson LH, Lamerdin JE, Brooks PJ (2009). "Acetaldehyde stimulates FANCD2 monoubiquitination, H2AX phosphorylation, and BRCA1 phosphorylation in human cells in vitro: implications for alcohol-related carcinogenesis". Mutat. Res. 664 (1–2): 77–83. doi:10.1016/j.mrfmmm.2009.03.011. PMC 2807731. PMID 19428384. Unknown parameter
|month=ignored (help) - ↑ Theruvathu JA, Jaruga P, Nath RG, Dizdaroglu M, Brooks PJ (2005). "Polyamines stimulate the formation of mutagenic 1,N2-propanodeoxyguanosine adducts from acetaldehyde". Nucleic Acids Res. 33 (11): 3513–20. doi:10.1093/nar/gki661. PMC 1156964. PMID 15972793.
- ↑ Friedenson B (2012). "Preventing hereditary cancers caused by opportunistic carcinogens". J Med Med Sci. 3: 160–178.
- ↑ Friedenson B, “Preventing hereditary cancers associated with BRCA1 and BRCA2 gene mutations” 2012
- ↑ Wooster R, Weber BL (2003). "Breast and ovarian cancer". N. Engl. J. Med. 348 (23): 2339–47. doi:10.1056/NEJMra012284. PMID 12788999. Unknown parameter
|month=ignored (help) - ↑ Coleman RE, Rubens RD (1987). "The clinical course of bone metastases from breast cancer". Br J Cancer. 55 (1): 61–6. PMC 2001575. PMID 3814476.
- ↑ Coleman RE, Seaman JJ (2001). "The role of zoledronic acid in cancer: clinical studies in the treatment and prevention of bone metastases". Semin Oncol. 28 (2 Suppl 6): 11–6. PMID 11346860.
- ↑ Chiang AC, Massagué J (2008). "Molecular basis of metastasis". N Engl J Med. 359 (26): 2814–23. doi:10.1056/NEJMra0805239. PMID 19109576.

