Bisphosphonate
|
WikiDoc Resources for Bisphosphonate |
|
Articles |
|---|
|
Most recent articles on Bisphosphonate Most cited articles on Bisphosphonate |
|
Media |
|
Powerpoint slides on Bisphosphonate |
|
Evidence Based Medicine |
|
Clinical Trials |
|
Ongoing Trials on Bisphosphonate at Clinical Trials.gov Trial results on Bisphosphonate Clinical Trials on Bisphosphonate at Google
|
|
Guidelines / Policies / Govt |
|
US National Guidelines Clearinghouse on Bisphosphonate NICE Guidance on Bisphosphonate
|
|
Books |
|
News |
|
Commentary |
|
Definitions |
|
Patient Resources / Community |
|
Patient resources on Bisphosphonate Discussion groups on Bisphosphonate Patient Handouts on Bisphosphonate Directions to Hospitals Treating Bisphosphonate Risk calculators and risk factors for Bisphosphonate
|
|
Healthcare Provider Resources |
|
Causes & Risk Factors for Bisphosphonate |
|
Continuing Medical Education (CME) |
|
International |
|
|
|
Business |
|
Experimental / Informatics |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]
Overview
In pharmacology, bisphosphonates (also called: diphosphonates) is a class of drugs that inhibits osteoclast action and the resorption of bone. Its uses include the prevention and treatment of osteoporosis, osteitis deformans ("Paget's disease of bone"), bone metastasis (with or without hypercalcemia), multiple myeloma and other conditions that feature bone fragility.
History
Bisphosphonates were developed in the 19th century, but were first investigated in the 1960s for use in disorders of bone metabolism. Their non-medical use included water softening in irrigation systems used in orange groves. The initial rationale for their use in humans was their potential in preventing the dissolution of hydroxylapatite, the principal bone mineral, and hence arresting bone loss. Only in the 1990s was their actual mechanism of action demonstrated.[1]
Chemistry and classes
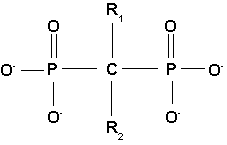
All bisphosphonate drugs share a common P-C-P "backbone":
The two PO3 (phosphate) groups covalently linked to carbon determine both the name "bisphosphonate" and the function of the drugs.
The long side chain (R2 in the diagram) determines the chemical properties, the mode of action and the strength of bisphosphonate drugs. The short side chain (R1), often called the 'hook,' mainly influences chemical properties and pharmacokinetics.
Pharmacokinetics
Of the bisphosphonate that is resorbed (from oral preparation) or infused (for intravenous drugs), about 50% is excreted unchanged by the kidney. The remainder has a very high affinity for bone tissue, and is rapidly absorbed onto the bone surface.
Mechanism of action
Bisphosphonates, when attached to bone tissue, are "ingested" by osteoclasts, the bone cell that breaks down bone tissue.
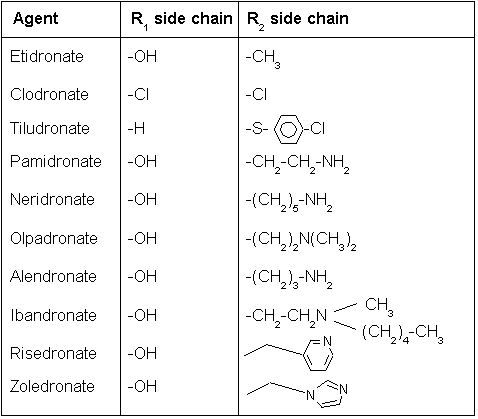
There are two classes of bisphosphonate: the N-containing and non-N-containing bisphosphonates. The two types of bisphosphonates work differently in killing osteoclast cells.
Non-nitrogenous
Non-N-containing bisphosphonates:
- Etidronate (Didronel®) - 1 (potency relative to that of etidronate)
- Clodronate (Bonefos®, Loron®) - 10
- Tiludronate (Skelid®) - 10
The non-nitrogenous bisphosphonates(disphosphonates) are metabolised in the cell to compounds that compete with adenosine triphosphate (ATP) in the cellular energy metabolism. The osteoclast initiates apoptosis and dies, leading to an overall decrease in the breakdown of bone.[2]
Nitrogenous
N-containing bisphosphonates:
- Pamidronate (APD, Aredia®) - 100
- Neridronate - 100
- Olpadronate - 500
- Alendronate (Fosamax®) - 500
- Ibandronate (Boniva®) - 1000
- Risedronate (Actonel®) - 2000
- Zoledronate (Zometa®) - 10000
Nitrogenous bisphosphonates act on bone metabolism by binding and blocking the enzyme farnesyl diphosphate synthase (FPPS) in the HMG-CoA reductase pathway (also known as the mevalonate pathway).[3]
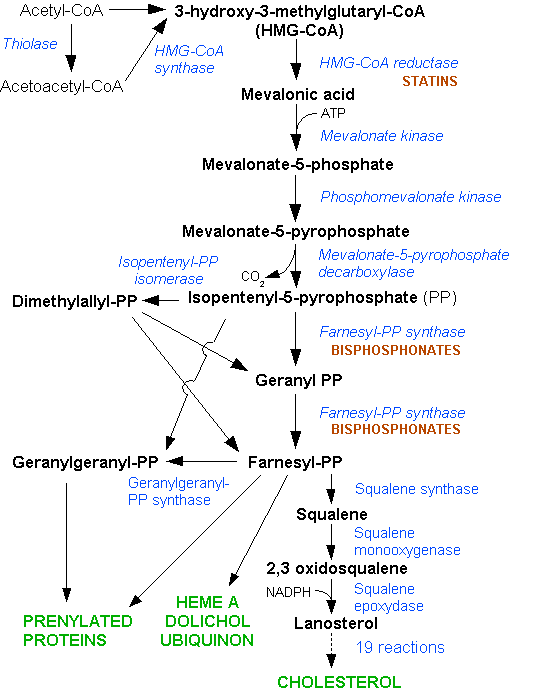
Disruption of the HMG CoA-reductase pathway at the level of FPPS prevents the formation of two metabolites (farnesol and geranylgeraniol) that are essential for connecting some small proteins to the cell membrane. This phenomenon is known as prenylation, and is important for proper sub-cellular protein trafficking (see "lipid anchored protein" for the principles of this phenomenon).[4]
While inhibition of protein prenylation may affect many proteins found in an osteoclast, disruption to the lipid modification of Ras, Rho, Rac proteins has been speculated to underlie the effects of bisphosphonates. These proteins can affect both osteoclastogenesis, cell survival, and cytoskeletal dynamics. In particular, the cytoskeleton is vital for maintaining the "ruffled border" that is required for contact between a resorbing osteoclast and a bone surface.
Statins are another class of drugs that inhibit the HMG-CoA reductase pathway. Unlike bisphosphonates, statins do not bind to bone surfaces with high affinity, and are thus not specific for bone. Nevertheless, some studies have reported a decreased rate of fracture (an indicator of osteoporosis) and/or an increased bone mineral density in statin users. The overall efficacy of statins in the treatment osteoporosis remains controversial.
Uses
Bisphosphonates are used clinically for the treatment of osteoporosis, osteitis deformans (Paget's disease of the bone), bone metastasis (with or without hypercalcemia), multiple myeloma and other conditions that feature bone fragility.
In osteoporosis and Paget's, alendronate and risedronate are the most popular first-line drugs. If these are ineffective or the patient develops digestive tract problems, intravenous pamidronate may be used. Alternatively, strontium ranelate or teriparatide are used for refractory disease, and the SERM raloxifene is occasionally administered in postmenopausal women instead of bisphosphonates.
High-potency intravenous bisphosphonates have shown to modify progression of skeletal metastasis in several forms of cancer, especially breast cancer.
Other bisphosphonates, medronate (R1, R2 = H) and oxidronate (R1 = H, R2 = OH) are mixed with radioactive technetium and are injected for imaging bone and detecting bone disease.
More recently, bisphosphonates have been used to reduce fracture rates in children with osteogenesis imperfecta.
Side-effects
- Oral bisphosphonates can give stomach upset and inflammation and erosions of the esophagus, which is the main problem of oral N-containing preparations. This can be prevented by remaining seated upright for 30 to 60 minutes after taking the medication.
- Intravenous bisphosphonates can give fever and flu-like symptoms after the first infusion, which is thought to occur because of their potential to activate human γδ T cells. Notably, these symptoms do not recur with subsequent infusions.
- There is a slightly increased risk for electrolyte disturbances, but not enough to warrant regular monitoring.
- In chronic renal failure, the drugs are excreted much slower, and dose adjustment is required.
- Bisphosphonates have been associated with osteonecrosis of the jaw; with the mandible twice as frequently affected as the maxilla and most cases occurring following high-dose intravenous administration used for some cancer patients. Some 60% of cases are preceded by a dental surgical procedure and it has been suggested that bisphosphonate treatment should be postponed until after any dental work to eliminate potential sites of infection.[5]
- A number of cases of severe bone, joint or musculoskeletal pain have been reported, prompting labeling changes[6]
Footnotes
- ↑ Fleisch H (2002). "Development of bisphosphonates". Breast Cancer Res. 4 (1): 30–4. PMID 11879557.
- ↑ Frith J, Mönkkönen J, Blackburn G, Russell R, Rogers M (1997). "Clodronate and liposome-encapsulated clodronate are metabolized to a toxic ATP analog, adenosine 5'-(beta, gamma-dichloromethylene) triphosphate, by mammalian cells in vitro". J Bone Miner Res. 12 (9): 1358–67. PMID 9286751.
- ↑ van Beek E, Cohen L, Leroy I, Ebetino F, Löwik C, Papapoulos S (2003). "Differentiating the mechanisms of antiresorptive action of nitrogen containing bisphosphonates". Bone. 33 (5): 805–11. PMID 14623056. Unknown parameter
|month=ignored (help) - ↑ van beek E, Löwik C, van der Pluijm G, Papapoulos S (1999). "The role of geranylgeranylation in bone resorption and its suppression by bisphosphonates in fetal bone explants in vitro: A clue to the mechanism of action of nitrogen-containing bisphosphonates". J Bone Miner Res. 14 (5): 722–9. PMID 10320520.
- ↑ Woo S, Hellstein J, Kalmar J (2006). "Narrative [corrected] review: bisphosphonates and osteonecrosis of the jaws". Ann Intern Med. 144 (10): 753–61. PMID 16702591.
- ↑ Wysowski D, Chang J (2005). "Alendronate and risedronate: reports of severe bone, joint, and muscle pain". Arch Intern Med. 165 (3): 346–7. PMID 15710802.