Aztreonam (injection): Difference between revisions
No edit summary |
No edit summary |
||
| Line 151: | Line 151: | ||
|useInPed=The safety and effectiveness of intravenous AZACTAM have been established in the age groups 9 months to 16 years. Use of AZACTAM in these age groups is supported by evidence from adequate and well-controlled studies of AZACTAM in adults with additional efficacy, safety, and pharmacokinetic data from noncomparative clinical studies in pediatric patients. Sufficient data are not available for pediatric patients under 9 months of age or for the following treatment indications/pathogens: septicemia and skin and skin-structure infections (where the skin infection is believed or known to be due to H. influenzae type b). In pediatric patients with cystic fibrosis, higher doses of AZACTAM may be warranted. (See CLINICAL PHARMACOLOGY, DOSAGE AND ADMINISTRATION, and CLINICAL STUDIES.) | |useInPed=The safety and effectiveness of intravenous AZACTAM have been established in the age groups 9 months to 16 years. Use of AZACTAM in these age groups is supported by evidence from adequate and well-controlled studies of AZACTAM in adults with additional efficacy, safety, and pharmacokinetic data from noncomparative clinical studies in pediatric patients. Sufficient data are not available for pediatric patients under 9 months of age or for the following treatment indications/pathogens: septicemia and skin and skin-structure infections (where the skin infection is believed or known to be due to H. influenzae type b). In pediatric patients with cystic fibrosis, higher doses of AZACTAM may be warranted. (See CLINICAL PHARMACOLOGY, DOSAGE AND ADMINISTRATION, and CLINICAL STUDIES.) | ||
|useInGeri=Renal status is a major determinant of dosage in the elderly; these patients in particular may have diminished renal function. Serum creatinine may not be an accurate determinant of renal status. Therefore, as with all antibiotics eliminated by the kidneys, estimates of creatinine clearance should be obtained and appropriate dosage modifications made if necessary. | |useInGeri=Renal status is a major determinant of dosage in the elderly; these patients in particular may have diminished renal function. Serum creatinine may not be an accurate determinant of renal status. Therefore, as with all antibiotics eliminated by the kidneys, estimates of creatinine clearance should be obtained and appropriate dosage modifications made if necessary. | ||
|useInRenalImpair=Prolonged serum levels of aztreonam may occur in patients with transient or persistent renal insufficiency. Therefore, the dosage of AZACTAM should be halved in patients with estimated creatinine clearances between 10 and 30 mL/min/1.73 m2 after an initial loading dose of 1 or 2 g. | |useInRenalImpair=Prolonged serum levels of aztreonam may occur in patients with transient or persistent renal insufficiency. Therefore, the dosage of AZACTAM should be halved in patients with estimated creatinine clearances between 10 and 30 mL/min/1.73 m2 after an initial loading dose of 1 or 2 g. | ||
| Line 178: | Line 177: | ||
*Lactated Ringer’s and 5% Dextrose Injection | *Lactated Ringer’s and 5% Dextrose Injection | ||
*Plasma-Lyte M and 5% Dextrose | *Plasma-Lyte M and 5% Dextrose | ||
|overdose=If necessary, aztreonam may be cleared from the serum by [[hemodialysis]] and/or [[peritoneal dialysis]]. | |||
|drugBox={{Drugbox2 | |||
| verifiedrevid = 458976305 | |||
| IUPAC_name = 2-({[(1''Z'')-1-(2-amino-1,3-thiazol-4-yl) -2- {[(2''S'',3''S'')-2-methyl-4-oxo-1-sulfoazetidin-3-yl]amino} -2- oxoethylidene]amino}oxy)-2-methylpropanoic acid | |||
| image = Aztreonam Structure.png | |||
| width = 150px | |||
<!--Clinical data--> | |||
| tradename = Azactam | |||
| Drugs.com = {{drugs.com|monograph|aztreonam}} | |||
| pregnancy_category = B1 <small>([[Australia|Au]])</small>, B <small>([[United States|U.S.]])</small> | |||
| legal_status = ℞-only <small>(U.S.)</small> | |||
| routes_of_administration = [[Intravenous therapy|Intravenous]], [[Intramuscular injection|intramuscular]], [[inhalation]] | |||
<!--Pharmacokinetic data--> | |||
| bioavailability = 100% ([[Intramuscular injection|IM]]) 0.1% (Oral in Rats) Unknown (Oral in humans) | |||
| protein_bound = 56% | |||
| metabolism = hepatic (minor %) | |||
| elimination_half-life = 1.7 hours | |||
| excretion = [[Kidney|Renal]] | |||
<!--Identifiers--> | |||
| CASNo_Ref = {{cascite}} | |||
| CAS_number_Ref = {{cascite|correct|??}} | |||
| CAS_number = 78110-38-0 | |||
| ATC_prefix = J01 | |||
| ATC_suffix = DF01 | |||
| ATC_supplemental = | |||
| PubChem = 54116 | |||
| DrugBank_Ref = {{drugbankcite|correct|drugbank}} | |||
| DrugBank = DB00355 | |||
| ChemSpiderID_Ref = {{chemspidercite|correct|chemspider}} | |||
| ChemSpiderID = 4674940 | |||
| UNII_Ref = {{fdacite|correct|FDA}} | |||
| UNII = G2B4VE5GH8 | |||
| KEGG_Ref = {{keggcite|correct|kegg}} | |||
| KEGG = D00240 | |||
| ChEBI_Ref = {{ebicite|correct|EBI}} | |||
| ChEBI = 161680 | |||
| ChEMBL_Ref = {{ebicite|correct|EBI}} | |||
| ChEMBL = 158 | |||
<!--Chemical data--> | |||
| C=13 | H=17 | N=5 | O=8 | S=2 | |||
| molecular_weight = 435.433 [[Gram|g]]/[[Mole (unit)|mol]] | |||
| smiles = O=S(=O)(O)N2C(=O)[C@@H](NC(=O)C(=N\OC(C(=O)O)(C)C)/c1nc(sc1)N)[C@@H]2C | |||
| InChI = 1/C13H17N5O8S2/c1-5-7(10(20)18(5)28(23,24)25)16-9(19)8(6-4-27-12(14)15-6)17-26-13(2,3)11(21)22/h4-5,7H,1-3H3,(H2,14,15)(H,16,19)(H,21,22)(H,23,24,25)/b17-8-/t5-,7-/m0/s1 | |||
| InChIKey = WZPBZJONDBGPKJ-VEHQQRBSBK | |||
| StdInChI_Ref = {{stdinchicite|correct|chemspider}} | |||
| StdInChI = 1S/C13H17N5O8S2/c1-5-7(10(20)18(5)28(23,24)25)16-9(19)8(6-4-27-12(14)15-6)17-26-13(2,3)11(21)22/h4-5,7H,1-3H3,(H2,14,15)(H,16,19)(H,21,22)(H,23,24,25)/b17-8-/t5-,7-/m0/s1 | |||
| StdInChIKey_Ref = {{stdinchicite|correct|chemspider}} | |||
| StdInChIKey = WZPBZJONDBGPKJ-VEHQQRBSSA-N | |||
}} | |||
|structure=Aztreonam is designated chemically as (Z)-2-[[[(2-amino-4-thiazolyl)[[(2S,3S)-2-methyl-4-oxo-1-sulfo-3-azetidinyl]carbamoyl]methylene]amino]oxy]-2-methylpropionic acid. Structural formula: | |||
[[file:Aztreonam Structure.png|none|350px]] | |||
|alcohol=Alcohol-Aztreonam interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication. | |alcohol=Alcohol-Aztreonam interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication. | ||
}} | }} | ||
Revision as of 17:54, 19 January 2015
{{DrugProjectFormSinglePage |authorTag=Alberto Plate [1] |genericName=Aztreonam |aOrAn=a |drugClass=monobactam antibiotic |indicationType=treatment |indication=urinary tract infections (complicated and uncomplicated), lower respiratory tract infections, septicemia, skin and skin-structure infections, intra-abdominal infections and gynecologic infections. AZACTAM is indicated for the treatment of the following infections caused by susceptible Gram-negative microorganisms:
Urinary Tract Infections (complicated and uncomplicated)
Including pyelonephritis and cystitis (initial and recurrent) caused by Escherichia coli, Klebsiella pneumoniae, Proteus mirabilis, Pseudomonas aeruginosa, Enterobacter cloacae, Klebsiella oxytoca, Citrobacter species, and Serratia marcescens.
Lower Respiratory Tract Infections
Including pneumonia and bronchitis caused by Escherichia coli, Klebsiella pneumoniae, Pseudomonas aeruginosa, Haemophilus influenzae, Proteus mirabilis, Enterobacter species, and Serratia marcescens.
Septicemia
Caused by Escherichia coli, Klebsiella pneumoniae, Pseudomonas aeruginosa, Proteus mirabilis, Serratia marcescens, and Enterobacter species.
Skin and Skin-Structure Infections
Including those associated with postoperative wounds, ulcers, and burns, caused by Escherichia coli, Proteus mirabilis, Serratia marcescens, Enterobacter species, Pseudomonas aeruginosa, Klebsiella pneumoniae, and Citrobacter species.
Intra-abdominal Infections
Including peritonitis caused by Escherichia coli, Klebsiella species including K. pneumoniae, Enterobacter species including E. cloacae, Pseudomonas aeruginosa, Citrobacter species* including C. freundii, and Serratia species* including S. marcescens.
Gynecologic Infections
Including endometritis and pelvic cellulitis caused by Escherichia coli, Klebsiella pneumoniae, Enterobacter species including E. cloacae, and Proteus mirabilis.
AZACTAM is indicated for adjunctive therapy to surgery in the management of infections caused by susceptible organisms, including abscesses, infections complicating hollow viscus perforations, cutaneous infections, and infections of serous surfaces. AZACTAM is effective against most of the commonly encountered Gram-negative aerobic pathogens seen in general surgery |adverseReactions=chest discomfort, abdominal pain, vomiting, alkaline phosphatase raised, ALT/SGPT level raised, AST/SGOT level raised, serum creatinine raised, cough, nasal congestion, Pain in throat, wheezing, fever. |blackBoxWarningTitle=TITLE |blackBoxWarningBody=Condition Name: (Content) |fdaLIADAdult=AZACTAM, an intravenous solution in GALAXY plastic containers (PL 2040), is intended for intravenous use only. Dosage should be determined by susceptibility of the causative organisms, severity and site of infection, and the condition of the patient.
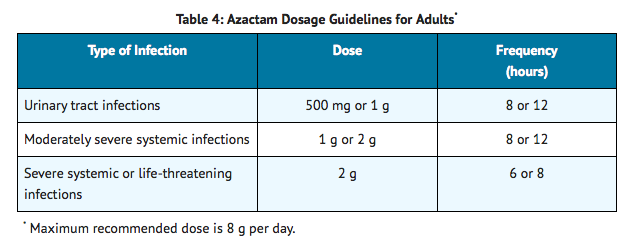
Because of the serious nature of infections due to Pseudomonas aeruginosa, dosage of 2 g every six or eight hours is recommended, at least upon initiation of therapy, in systemic infections caused by this organism.
The intravenous route is recommended for patients requiring single doses greater than 1 g or those with bacterial septicemia, localized parenchymal abscess (eg, intra-abdominal abscess), peritonitis, or other severe systemic or life-threatening infections.
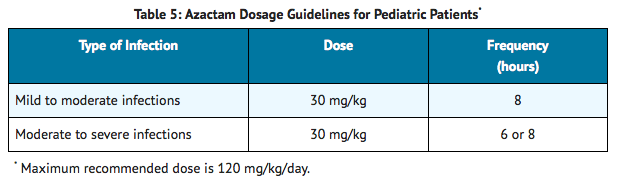
The duration of therapy depends on the severity of infection. Generally, AZACTAM should be continued for at least 48 hours after the patient becomes asymptomatic or evidence of bacterial eradication has been obtained. Persistent infections may require treatment for several weeks. Doses smaller than those indicated should not be used. |offLabelAdultGuideSupport=There is limited information regarding Off-Label Guideline-Supported Use of Aztreonam in adult patients. |offLabelAdultNoGuideSupport=There is limited information regarding Off-Label Non–Guideline-Supported Use of Aztreonam in adult patients. |fdaLIADPed=AZACTAM should be administered intravenously to pediatric patients with normal renal function. There are insufficient data regarding intramuscular administration to pediatric patients or dosing in pediatric patients with renal impairment.
|offLabelPedGuideSupport=There is limited information regarding Off-Label Guideline-Supported Use of Aztreonam in pediatric patients. |offLabelPedNoGuideSupport=There is limited information regarding Off-Label Non–Guideline-Supported Use of Aztreonam in pediatric patients. |contraindications=This preparation is contraindicated in patients with known hypersensitivity to aztreonam or any other component in the formulation. |warnings=Both animal and human data suggest that AZACTAM (aztreonam injection) is rarely cross-reactive with other beta-lactam antibiotics and weakly immunogenic. Treatment with aztreonam can result in hypersensitivity reactions in patients with or without prior exposure. Careful inquiry should be made to determine whether the patient has any history of hypersensitivity reactions to any allergens.
While cross-reactivity of aztreonam with other beta-lactam antibiotics is rare, this drug should be administered with caution to any patient with a history of hypersensitivity to beta-lactams (eg, penicillins, cephalosporins, and/or carbapenems). Treatment with aztreonam can result in hypersensitivity reactions in patients with or without prior exposure to aztreonam. If an allergic reaction to aztreonam occurs, discontinue the drug and institute supportive treatment as appropriate (eg, maintenance of ventilation, pressor amines, antihistamines, corticosteroids). Serious hypersensitivity reactions may require epinephrine and other emergency measures.
Clostridium difficile–associated diarrhea (CDAD) has been reported with use of nearly all antibacterial agents, including AZACTAM, and may range in severity from mild diarrhea to fatal colitis. Treatment with antibacterial agents alters the normal flora of the colon leading to overgrowth of C. difficile.
C. difficile produces toxins A and B which contribute to the development of CDAD. Hypertoxin-producing strains of C. difficile cause increased morbidity and mortality, as these infections can be refractory to antimicrobial therapy and may require colectomy. CDAD must be considered in all patients who present with diarrhea following antibiotic use. Careful medical history is necessary since CDAD has been reported to occur over 2 months after the administration of antibacterial agents.
If CDAD is suspected or confirmed, ongoing antibiotic use not directed against C. difficile may need to be discontinued. Appropriate fluid and electrolyte management, protein supplementation, antibiotic treatment of C. difficile, and surgical evaluation should be instituted as clinically indicated.
Rare cases of toxic epidermal necrolysis have been reported in association with aztreonam in patients undergoing bone marrow transplant with multiple risk factors including sepsis, radiation therapy, and other concomitantly administered drugs associated with toxic epidermal necrolysis. |clinicalTrials=Local reactions (eg, phlebitis/thrombophlebitis; discomfort/swelling) following intravenous administration occurred at rates of approximately 1.9%.
Systemic reactions (considered to be related to therapy or of uncertain etiology) occurring at an incidence of 1% to 1.3% include diarrhea, nausea and/or vomiting, and rash. Reactions occurring at an incidence of less than 1% are listed within each body system in order of decreasing severity:
Hypersensitivity
Hematologic
Gastrointestinal
- Abdominal cramps
- CDAD
- Onset of pseudomembranous colitis symptoms may occur during or after antibiotic treatment.
Dermatologic
- Toxic epidermal necrolysis
- Purpura
- Erythema multiforme
- Exfoliative dermatitis
- urticaria
- Petechiae
- Pruritus
- Diaphoresis
Cardiovascular
- Hypotension
- Transient ECG changes (ventricular bigeminy and PVC)
- Flushing
Respiratory
Hepatobiliary
Nervous System
Musculoskeletal
Special Senses
Other
Body as a Whole
Pediatric Adverse Reactions
Of the 612 pediatric patients who were treated with AZACTAM in clinical trials, less than 1% required discontinuation of therapy due to adverse events. The following systemic adverse events, regardless of drug relationship, occurred in at least 1% of treated patients in domestic clinical trials: rash (4.3%), diarrhea (1.4%), and fever (1.0%). These adverse events were comparable to those observed in adult clinical trials.
In 343 pediatric patients receiving intravenous therapy, the following local reactions were noted: pain (12%), erythema (2.9%), induration (0.9%), and phlebitis (2.1%). In the US patient population, pain occurred in 1.5% of patients, while each of the remaining 3 local reactions had an incidence of 0.5%.
The following laboratory adverse events, regardless of drug relationship, occurred in at least 1% of treated patients: increased eosinophils (6.3%), increased platelets (3.6%), neutropenia (3.2%), increased AST (3.8%), increased ALT (6.5%), and increased serum creatinine (5.8%).
In US pediatric clinical trials, neutropenia (absolute neutrophil count less than 1000/mm3) occurred in 11.3% of patients (8/71) younger than 2 years receiving 30 mg/kg every 6 hours. AST and ALT elevations to greater than 3 times the upper limit of normal were noted in 15% to 20% of patients aged 2 years or above receiving 50 mg/kg every 6 hours. The increased frequency of these reported laboratory adverse events may be due to either increased severity of illness treated or higher doses of AZACTAM administered. |drugInteractions=Concomitant administration of probenecid or furosemide and aztreonam causes clinically insignificant increases in the serum levels of aztreonam. Single-dose intravenous pharmacokinetic studies have not shown any significant interaction between aztreonam and concomitantly administered gentamicin, nafcillin sodium, cephradine, clindamycin, or metronidazole. No reports of disulfiram-like reactions with alcohol ingestion have been noted; this is not unexpected since aztreonam does not contain a methyl-tetrazole side chain. |FDAPregCat=B |useInPregnancyFDA=In pregnant women, aztreonam crosses the placenta and enters the fetal circulation.
Developmental toxicity studies in pregnant rats and rabbits with daily doses of aztreonam up to 1800 and 1200 mg/kg, respectively, revealed no evidence of embryotoxicity or fetotoxicity or teratogenicity. These doses, based on body surface area, are 2.2- and 2.9-fold greater than the MRHD for adults of 8 g per day. A peri/postnatal study in rats revealed no drug-induced changes in any maternal, fetal, or neonatal parameters. The highest dose used in this study, 1800 mg/kg/day, is 2.2 times the MRHD based on body surface area.
There are no adequate and well-controlled studies of aztreonam on human pregnancy outcomes. Because animal reproduction studies are not always predictive of human response, aztreonam should be used during pregnancy only if clearly needed. |useInNursing=Aztreonam is excreted in human milk in concentrations that are less than 1% of concentrations determined in simultaneously obtained maternal serum; consideration should be given to temporary discontinuation of nursing and use of formula feedings. |useInPed=The safety and effectiveness of intravenous AZACTAM have been established in the age groups 9 months to 16 years. Use of AZACTAM in these age groups is supported by evidence from adequate and well-controlled studies of AZACTAM in adults with additional efficacy, safety, and pharmacokinetic data from noncomparative clinical studies in pediatric patients. Sufficient data are not available for pediatric patients under 9 months of age or for the following treatment indications/pathogens: septicemia and skin and skin-structure infections (where the skin infection is believed or known to be due to H. influenzae type b). In pediatric patients with cystic fibrosis, higher doses of AZACTAM may be warranted. (See CLINICAL PHARMACOLOGY, DOSAGE AND ADMINISTRATION, and CLINICAL STUDIES.) |useInGeri=Renal status is a major determinant of dosage in the elderly; these patients in particular may have diminished renal function. Serum creatinine may not be an accurate determinant of renal status. Therefore, as with all antibiotics eliminated by the kidneys, estimates of creatinine clearance should be obtained and appropriate dosage modifications made if necessary. |useInRenalImpair=Prolonged serum levels of aztreonam may occur in patients with transient or persistent renal insufficiency. Therefore, the dosage of AZACTAM should be halved in patients with estimated creatinine clearances between 10 and 30 mL/min/1.73 m2 after an initial loading dose of 1 or 2 g.
When only the serum creatinine concentration is available, the following formula (based on sex, weight, and age of the patient) may be used to approximate the creatinine clearance (Clcr). The serum creatinine should represent a steady state of renal function.

In patients with severe renal failure (creatinine clearance less than 10 mL/min/1.73 m2), such as those supported by hemodialysis, the usual dose of 500 mg, 1 g, or 2 g should be given initially. The maintenance dose should be one-fourth of the usual initial dose given at the usual fixed interval of 6, 8, or 12 hours. For serious or life-threatening infections, in addition to the maintenance doses, one-eighth of the initial dose should be given after each hemodialysis session. |IVCompat=Infusion of AZACTAM should be completed within a 20- to 60-minute period. The plastic container is a single-dose unit; discard any unused portion remaining in the container.
The following infusion solutions are compatible with AZACTAM (aztreonam injection) in GALAXY plastic container (PL 2040):
- Sodium Chloride Injection, USP, 0.9%
- Ringer’s Injection, USP
- Lactated Ringer’s Injection, USP
- Dextrose Injection, USP, 5% or 10%
- Dextrose and Sodium Chloride Injection, USP, 5%:0.9%, 5%:0.45%, or 5%:0.2%
- Sodium Lactate Injection, USP (M/6 Sodium Lactate)
- Ionosol ® B and 5% Dextrose
- Isolyte ® E
- Isolyte ® E with 5% Dextrose
- Isolyte ® M with 5% Dextrose
- Normosol ®-R
- Normosol ®-R and 5% Dextrose
- Normosol ®-M and 5% Dextrose
- Mannitol Injection, USP, 5% or 10%
- Lactated Ringer’s and 5% Dextrose Injection
- Plasma-Lyte M and 5% Dextrose
|overdose=If necessary, aztreonam may be cleared from the serum by hemodialysis and/or peritoneal dialysis. |drugBox=
|structure=Aztreonam is designated chemically as (Z)-2-[[[(2-amino-4-thiazolyl)[[(2S,3S)-2-methyl-4-oxo-1-sulfo-3-azetidinyl]carbamoyl]methylene]amino]oxy]-2-methylpropionic acid. Structural formula:
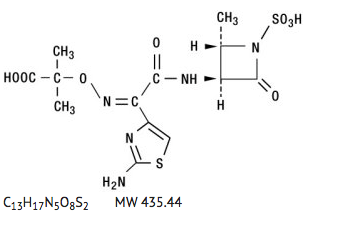
|alcohol=Alcohol-Aztreonam interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication. }}
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [2]; Associate Editor(s)-in-Chief: Chetan Lokhande, M.B.B.S [3]
Overview
Aztreonam' (Azactam®) is a synthetic monocyclic beta-lactam antibiotic (a monobactam) originally isolated from Chromobacterium violaceum. It was approved by the FDA in 1986. It is resistant to some beta-lactamases, but is inactivated by extended-spectrum beta-lactamases.
Category
Monobactam
US Brand Names
AZACTAM®
FDA Package Insert
Description | Clinical Pharmacology | Microbiology | Indications and Usage | Contraindications | Warnings and Precautions | Adverse Reactions | Drug Interactions | Overdosage | Clinical Studies | Dosage and Administration | How Supplied | Labels and Packages
Mechanism of Action
Aztreonam is similar in action to penicillin. It inhibits mucopeptide synthesis in the bacterial cell wall. It has a very high affinity for penicillin-binding protein 3 (PBP-3) and mild affinity for PBP-1a. Aztreonam binds the penicillin-binding proteins of gram-positive and anaerobic bacteria very poorly and is largely ineffective against them.[1] Aztreonam is bactericidal but less so than some of the cephalosporins.
References
- ↑ AHFS DRUG INFORMATION® 2006 (2006 ed ed.). American Society of Health-System Pharmacists. 2006.