Anaplastic large cell lymphoma pathophysiology
|
Anaplastic large cell lymphoma Microchapters |
|
Differentiating Anaplastic large cell lymphoma from other Diseases |
|---|
|
Diagnosis |
|
Treatment |
|
Case Studies |
|
Anaplastic large cell lymphoma pathophysiology On the Web |
|
American Roentgen Ray Society Images of Anaplastic large cell lymphoma pathophysiology |
|
Directions to Hospitals Treating Anaplastic large cell lymphoma |
|
Risk calculators and risk factors for Anaplastic large cell lymphoma pathophysiology |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [11];Associate Editor(s)-in-Chief: Shivali Marketkar, M.B.B.S. [12]Sowminya Arikapudi, M.B,B.S. [13]
Overview
Genetics
Molecular Biology
The majority of cases, greater than 90%, contain a clonal rearrangement of the T-cell receptor. This may be identified using PCR techniques, such as T-gamma multiplex PCR. Oncogenetic potential is conferred by upregulation of a tyrosine kinase gene on chromosome 2. Several different translocations involving this gene have been identified in different cases of this lymphoma. The most common is a chromosomal translocation involving the nucleophosmin gene on chromosome 5. The translocation may be identified by analysis of giemsa-banded metaphase spreads of tumour cells and is characterised by t(2;5)(p23;q35). The product of this fusion gene may be identified by immunohistochemistry using antiserum to ALK protein. Probes are available to identify the translocation by fluorescent in situ hybridization. The nucleophosmin component associated with the commonest translocation results in nuclear positivity as well as cytoplasmic positivity. Positivity with the other translocations may be confined to the cytoplasm.
Immunophenotype
The hallmark cells (and variants) show immunopositivity for CD30 (also known as Ki-1). True positivity requires localisation of signal to the cell membrane and/or paranuclear region (cyptolasmic positivity is considered non-specific and non-informative). Another useful marker which helps to differentiate this lesion from Hodgkin lymphoma is Clusterin. The neoplastic cells have a golgi staining pattern (hence paranuclear staining), which is characteristic of this lymphoma. The cells are also typically positive for a subset of markers of T-cell lineage. However, as with other T-cell lymphomas, they are usually negative for the pan T-cell marker CD3. Occasional examples are of null (neither T nor B) cell type. These lymphomas show immunopositivity for ALK protein in 70% of cases. They are also typically positive for EMA. In contrast to many B-cell anaplastic CD30 positive lymphomas, they are negative for markers of Epstein-Barr Virus (EBV).==Pathophysiology==
Genetics
Clonal T-cell receptor gene rearrangements are detected in 75% of cases[1], and immunoglobin gene rearrangements are seen in 10% of cases, and these cases are believed to be due to expanded EBV-driven B-cell populations.[2] Similarly, EBV-related sequences can be detected most cases, usually in B-cells but occasionally in T-cells.[3][4]. Trisomy 3, trisomy 5, and +X are the most frequent chromosomal abnormalities found in cases.[5][6]
Gross Pathology
The normal architecture of a lymph node is partially effaced by a polymorphous infiltrate and residual follicles are commonly seen. The polymorphous infiltrate consists of lymphocytes of moderate size with pale/clear cytoplasm and smaller reactive lymphocytes, eosinophils, histiocytes, plasma cells, and follicular dendritic cells. In addition, blast-like B-cells are occasionally seen. A classic morphological finding is the aborization and proliferation of high endothelial venules.[7] Hyperplastic germinal centers and Reed-Sternberg cells can also be seen.[8][9]
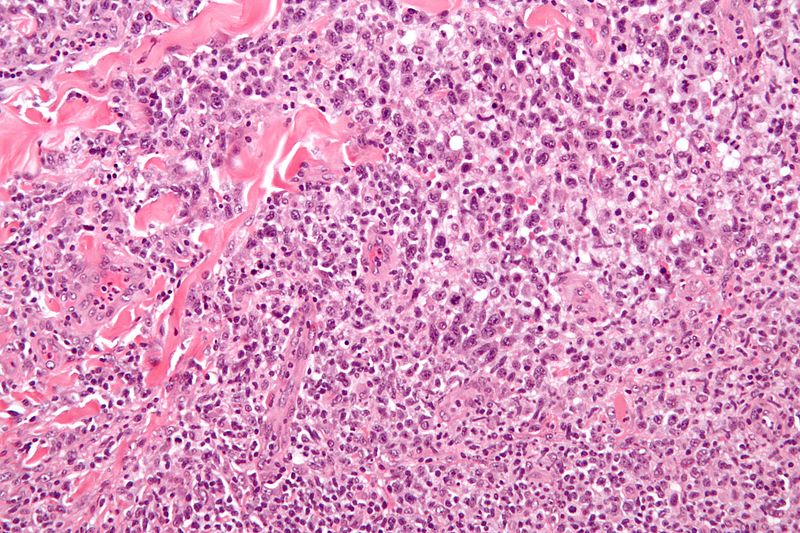
Microscopic Pathology
AILT typically has the phenotype of a mixture of CD4+ and CD8+ T-cells, with a CD4:CD8 ratio greater than unity. Polyclonal plasma cells and CD21+ follicular dendritic cells are also seen.[7] Due to the systemic nature of this disease, neoplastic cells can be found in lymph nodes, liver, spleen, skin, and bone marrow.
Causes
AILT was originally thought to be a premalignant condition, termed angioimmunoblastic lymphadenopathy, and this atypical reactive lymphadenopathy carried a risk for transformation into a lymphoma. Currently, it is postulated that the originating cell for this disease is a mature (post-thymic) CD4+ T-cell that arises de novo, although some researchers argue that there is a premalignant subtype of this disease.[10][11] The Epstein Barr virus (EBV) is observed in the majority of cases, and the virus has been found in the reactive B-cells that comprise part of the polymorphous infiltrate of this disease[3] and in the neoplastic T-cells.[4] Immunodeficiency is also seen with this disease, but it is thought to be a sequela to the condition and not a predisposing factor.
Microscopic Pathology
The hallmark cells are of medium size and feature abundant cytoplasm (which may be clear, amphophilic or eosinophilic), kidney shaped nuclei, and a paranuclear eosinophilic region. Occasional cells may be identified in which the plane of section passes through the nucleus in such a way that it appears to enclose a region of cytoplasm within a ring; such cells are called "doughnut" cells. The morphologic features of ALCL are variable. There are five morphological patterns:[12]
- "Common" pattern: This is the most common morphological variant (75%).[13] The cytoplasm may be either basophilic or eosinophilic and the cell might have many nuclei with dispersed or clumped chromatin. In large cells, nucleoli tend to be more prominent. Given that the lymphomatous cells grow in the lymph node's sinuses, this variant may resemble a metastatic tumor.
- Lymphohistiocytic pattern (10%): Histiocytes have an acidophilic cytoplasm and a perinuclear clear area, with an eccentric nuclei and condensed chromatin.[14] Lymphomatous cells cluster around the perivascular area as demonstrated by immunostaining with CD30 and ALK antibodies.[12]
- Hodgkin's like pattern (3.3%): The morphological characteristics of this pattern are similar to the nodular sclerosis variant of Hodgkin's lymphoma.[15] This pattern is predominately more common among female. There are two immunophenotype:[15]
- Small cell pattern (8.3%): Cells have nuclear irregularity and perivascular/intravascular distribution.[16] Occasionally, lymphomatous cells have a pale cytoplasm with a central nucleus, described as "fried egg cell".[12]
- Giant cell pattern (3.3%)
One single patient may present more than one morphologic pattern in the same or progressive biopsies[17].
Video
{{#ev:youtube|3-ajNCAGP4Y}}
References
- ↑ [1] Feller AC, Griesser H, Schilling CV, Wacker HH, Dallenbach F, Bartels H, Kuse R, Mak TW, Lennert K. "Clonal gene rearrangement patterns correlate with immunophenotype and clinical parameters in patients with angioimmunoblastic lymphadenopathy." Am J Pathol. 1988 Dec;133(3):549-56. PMID: 2849301
- ↑ [2] Lipford EH, Smith HR, Pittaluga S, Jaffe ES, Steinberg AD, Cossman J. "Clonality of angioimmunoblastic lymphadenopathy and implications for its evolution to malignant lymphoma." J Clin Invest. 1987 Feb;79(2):637-42. PMID: 3805286
- ↑ 3.0 3.1 [3] Weiss LM, Jaffe ES, Liu XF, Chen YY, Shibata D, Medeiros LJ. "Detection and localization of Epstein-Barr viral genomes in angioimmunoblastic lymphadenopathy and angioimmunoblastic lymphadenopathy-like lymphoma." Blood. 1992 Apr 1;79(7):1789-95. PMID: 1373088
- ↑ 4.0 4.1 [4] Anagnostopoulos I, Hummel M, Finn T, Tiemann M, Korbjuhn P, Dimmler C, Gatter K, Dallenbach F, Parwaresch MR, Stein H. "Heterogeneous Epstein-Barr virus infection patterns in peripheral T-cell lymphoma of angioimmunoblastic lymphadenopathy type."Blood. 1992 Oct 1;80(7):1804-12. PMID: 1327284
- ↑ [5] Kaneko Y, Maseki N, Sakurai M, Takayama S, Nanba K, Kikuchi M, Frizzera G. "Characteristic karyotypic pattern in T-cell lymphoproliferative disorders with reactive "angioimmunoblastic lymphadenopathy with dysproteinemia-type" features." Blood. 1988 Aug;72(2):413-21. PMID: 3261178
- ↑ [6] Schlegelberger B, Zhang Y, Weber-Matthiesen K, Grote W. "Detection of aberrant clones in nearly all cases of angioimmunoblastic lymphadenopathy with dysproteinemia-type T-cell lymphoma by combined interphase and metaphase cytogenetics." Blood. 1994 Oct 15;84(8):2640-8. PMID: 7919378
- ↑ 7.0 7.1
- ↑ [7] Quintanilla-Martinez L, Fend F, Moguel LR, Spilove L, Beaty MW, Kingma DW, Raffeld M, Jaffe ES. "Peripheral T-cell lymphoma with Reed-Sternberg-like cells of B-cell phenotype and genotype associated with Epstein-Barr virus infection." Am J Surg Pathol. 1999 Oct;23(10):1233-40. PMID: 10524524
- ↑ [8] Ree HJ, Kadin ME, Kikuchi M, Ko YH, Go JH, Suzumiya J, Kim DS. "Angioimmunoblastic lymphoma (AILD-type T-cell lymphoma) with hyperplastic germinal centers." Am J Surg Pathol. 1998 Jun;22(6):643-55. PMID: 9630171
- ↑ [9] Frizzera G, Kaneko Y, Sakurai M. "Angioimmunoblastic lymphadenopathy and related disorders: a retrospective look in search of definitions." Leukemia. 1989 Jan;3(1):1-5. PMID: 2642571
- ↑ [10] Smith JL, Hodges E, Quin CT, McCarthy KP, Wright DH. "Frequent T and B cell oligoclones in histologically and immunophenotypically characterized angioimmunoblastic lymphadenopathy." Am J Pathol. 2000 Feb;156(2):661-9. PMID: 10666395
- ↑ 12.0 12.1 12.2 Swerdlow, Steven (2008). WHO classification of tumours of haematopoietic and lymphoid tissues. Lyon, France: International Agency for Research on Cancer. ISBN 9789283224310.
- ↑ Falini B, Bigerna B, Fizzotti M, Pulford K, Pileri SA, Delsol G; et al. (1998). "ALK expression defines a distinct group of T/null lymphomas ("ALK lymphomas") with a wide morphological spectrum". Am J Pathol. 153 (3): 875–86. doi:10.1016/S0002-9440(10)65629-5. PMC 1853018. PMID 9736036.
- ↑ "Frequent Expression ofthe NPM-ALK Chimeric Fusion Protein inAnaplastic Large-Cell Lymphoma, Lympho-Histiocytic Type" (PDF).
- ↑ 15.0 15.1 Vassallo J, Lamant L, Brugieres L, Gaillard F, Campo E, Brousset P; et al. (2006). "ALK-positive anaplastic large cell lymphoma mimicking nodular sclerosis Hodgkin's lymphoma: report of 10 cases". Am J Surg Pathol. 30 (2): 223–9. PMID 16434897.
- ↑ Kinney MC, Collins RD, Greer JP, Whitlock JA, Sioutos N, Kadin ME (1993). "A small-cell-predominant variant of primary Ki-1 (CD30)+ T-cell lymphoma". Am J Surg Pathol. 17 (9): 859–68. PMID 8394652.
- ↑ Benharroch D, Meguerian-Bedoyan Z, Lamant L, Amin C, Brugières L, Terrier-Lacombe MJ; et al. (1998). "ALK-positive lymphoma: a single disease with a broad spectrum of morphology". Blood. 91 (6): 2076–84. PMID 9490693.