Dicloxacillin
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Rabin Bista, M.B.B.S. [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Dicloxacillin is a Beta-Lactam antibiotic that is FDA approved for the treatment of infections caused by penicillinase-producing staphylococci. Common adverse reactions include Dirarrhoea, Nausea, Vomiting.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Indications
- The penicillinase-resistant penicillins are indicated in the treatment of infections caused by penicillinase-producing staphylococci which have demonstrated susceptibility to the drugs. Cultures and susceptibility tests should be performed initially to determine the causative organism and their sensitivity to the drug .
- The penicillinase-resistant penicillins may be used to initiate therapy in suspected cases of resistant staphylococcal infections prior to the availability of laboratory test results. The penicillinase-resistant penicillins should not be used in infections caused by organisms susceptible to penicillin G. If the susceptibility tests indicate that the infection is due to an organism other than a resistant staphylococcus, therapy should not be continued with a penicillinase-resistant penicillin.
- To reduce the development of drug-resistant bacteria and maintain the effectiveness of dicloxacillin sodium capsules and other antibacterial drugs, dicloxacillin sodium capsules should be used only to treat or prevent infections that are proven or strongly suspected to be caused by susceptible bacteria. When culture and susceptibility information are available, they should be considered in selecting or modifying antibacterial therapy. In the absence of such data, local epidemiology and susceptibility patterns may contribute to the empiric selection of therapy.
Dosage
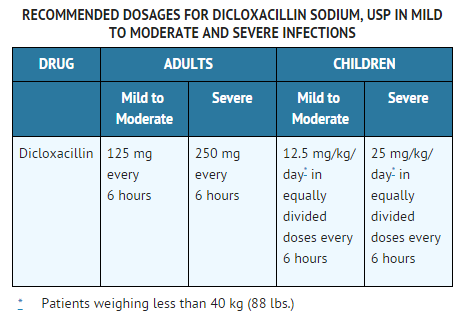
- The penicillinase-resistant penicillins are available for oral administration and for intramuscular and intravenous injection. The sodium salts of methicillin, oxacillin, and nafcillin may be administered parenterally and the sodium salts of cloxacillin, dicloxacillin, oxacillin, and nafcillin are available for oral use.
- Bacteriologic studies to determine the causative organisms and their sensitivity to the penicillinase-resistant penicillins should always be performed. Duration of therapy varies with the type and severity of infection as well as the overall condition of the patient, therefore, it should be determined by the clinical and bacteriological response of the patient. In severe staphylococcal infections, therapy with penicillinase-resistant penicillins should be continued for at least 14 days. Therapy should be continued for at least 48 hours after the patient has become afebrile, asymptomatic, and cultures are negative. The treatment of endocarditis and osteomyelitis may require a longer term of therapy.
- Concurrent administration of the penicillinase-resistant penicillins and probenecid increases and prolongs serum penicillin levels. Probenecid decreases the apparent volume of distribution and slows the rate of excretion by competitively inhibiting renal tubular secretion of penicillin. Penicillin-probenecid therapy is generally limited to those infections where very high serum levels of penicillin are necessary.
- Oral preparations of the penicillinase-resistant penicillins should not be used as initial therapy in serious, life-threatening infections. Oral therapy with the penicillinase-resistant penicillins may be used to follow-up the previous use of a parenteral agent as soon as the clinical condition warrants. For intramuscular gluteal injections, care should be taken to avoid sciatic nerve injury. With intravenous administration, particularly in elderly patients, care should be taken because of the possibility of thrombophlebitis.
- Dicloxacillin is best absorbed when taken on an empty stomach, and should be administered at least 1 hour before or 2 hours after meals. Dicloxacillin should be taken with at least 4 fluid ounces (120 mL) of water and should not be taken in the supine position or immediately before going to bed
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Dicloxacillin in adult patients.
Non–Guideline-Supported Use
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
There is limited information regarding Dicloxacillin FDA-Labeled Indications and Dosage (Pediatric) in the drug label.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Dicloxacillin in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Dicloxacillin in pediatric patients.
Contraindications
- Dicloxacillin sodium is contraindicated in persons who have shown hypersensitivity to any of the penicillins or any component of the formulations.
Warnings
- Serious and occasionally fatal hypersensitivity (anaphylactic shock with collapse) reactions have occurred in patients receiving penicillin. The incidence of anaphylactic shock in all penicillin-treated patients is between 0.015 and 0.04 percent. Anaphylactic shock resulting in death has occurred in approximately 0.002 percent of the patients treated. Although anaphylaxis is more frequent following a parenteral administration, it has occurred in patients receiving oral penicillins.
- When penicillin therapy is indicated, it should be initiated only after a comprehensive patient drug and allergy history has been obtained. If an allergic reaction occurs, the drug should be discontinued and the patient should receive supportive treatment, eg, artificial maintenance of ventilation, pressor amines, antihistamines, and corticosteroids. Individuals with a history of penicillin hypersensitivity may also experience allergic reactions when treated with a cephalosporin.
- Clostridium difficile associated diarrhea (CDAD) has been reported with use of nearly all antibacterial agents, including dicloxacillin sodium, and may range in severity from mild diarrhea to fatal colitis. Treatment with antibacterial agents alters the normal flora of the colon leading to overgrowth of C. difficile.
- C. difficile produces toxins A and B which contribute to the development of CDAD. Hypertoxin producing strains of C. difficile cause increased morbidity and mortality, as these infections can be refractory to antimicrobial therapy and may require colectomy. CDAD must be considered in all patients who present with diarrhea following antibiotic use. Careful medical history is necessary since CDAD has been reported to occur over two months after the administration of antibacterial agents.
- If CDAD is suspected or confirmed, ongoing antibiotic use not directed against C. difficile may need to be discontinued. Appropriate fluid and electrolyte management, protein supplementation, antibiotic treatment of C. difficile, and surgical evaluation should be instituted as clinically indicated.
Precautions
General
- Penicillinase-resistant penicillins should generally not be administered to patients with a history of sensitivity to any penicillin.
- Penicillin should be used with caution in individuals with histories of significant allergies and/or asthma. There is clinical and laboratory evidence of partial cross-allergenicity among penicillins and other β-lactam antibiotics including cephalosporins, cephamycins, and other 1-oxa-β-lactams. Whenever allergic reactions occur, penicillin should be withdrawn unless, in the opinion of the physician, the condition being treated is life-threatening and amenable only to penicillin therapy.
- The oral route of administration should not be relied upon in patients with severe illness, or with nausea, vomiting, gastric dilation, cardiospasm, or intestinal hypermotility. Occasionally patients will not absorb therapeutic amounts of orally administered penicillin.
- Prescribing dicloxacillin sodium capsules in the absence of a proven or strongly suspected bacterial infection or a prophylactic indication is unlikely to provide benefit to the patient and increases the risk of the development of drug-resistant bacteria.
Laboratory Tests
- Bacteriologic studies to determine the causative organisms and their susceptibility to the penicillinase-resistant penicillins should be performed. In the treatment of suspected staphylococcal infections, therapy should be changed to another active agent if culture tests fail to demonstrate the presence of staphylococci.
- Periodic assessment of organ system function including renal, hepatic, and hematopoietic should be made during prolonged therapy with the penicillinase-resistant penicillins.
- Blood cultures, white blood cell, and differential cell counts should be obtained prior to initiation of therapy and at least weekly during therapy with penicillinase-resistant penicillins.
- Periodic urinalysis, blood urea nitrogen, and creatinine determinations should be performed during therapy with the penicillinase-resistant penicillins and dosage alterations should be considered if these values become elevated. If any impairment of renal function is suspected or known to exist, a reduction in the total dosage should be considered and blood levels monitored to avoid possible neurotoxic reactions .
- AST (SGOT) and ALT(SGPT) values should be obtained periodically during therapy to monitor for possible liver function abnormalities.
Adverse Reactions
Clinical Trials Experience
Hypersensitive Reactions
- The reported incidence of allergic reactions to penicillin ranges from 0.7 to 10 percent. Sensitization is usually the result of treatment but some individuals have had immediate reactions to penicillin when first treated. In such cases, it is thought that the patients may have had prior exposure to the drug via trace amounts present in milk and vaccines.
- Two types of allergic reactions to penicillin are noted clinically, immediate and delayed.
- Immediate reactions usually occur within 20 minutes of administration and range in severity from urticaria and pruritus to angioneurotic edema, laryngospasm, bronchospasm, hypotension, vascular collapse, and death. Such immediate anaphylactic reactions are very rare and usually occur after parenteral therapy but have occurred in patients receiving oral therapy. Another type of immediate reaction, an accelerated reaction, may occur between 20 minutes and 48 hours after administration and may include urticaria, pruritus, and fever. Although laryngeal edema, laryngospasm, and hypotension occasionally occur, fatality is uncommon.
- Delayed allergic reactions to penicillin therapy usually occur after 48 hours and sometimes as late as 2 to 4 weeks after initiation of therapy. Manifestations of this type of reaction include serum sickness-like symptoms (ie, fever, malaise, urticaria, myalgia, arthralgia, abdominal pain) and various skin rashes.
Gastrointestinal Reactions
- Nausea, vomiting, diarrhea, stomatitis, black or hairy tongue, and other symptoms of gastrointestinal irritation may occur, especially during oral penicillin therapy.
- Pseudomembranous colitis has been reported with the use of dicloxacillin. Therefore, it is important to consider its diagnosis in patients who develop diarrhea in association with dicloxacillin use.
- Rare reports have been received during postmarketing surveillance of esophageal burning, esophagitis, and esophageal ulceration, particularly after ingestion of dicloxacillin capsules with an insufficient quantity of water and/or before going to bed.
Nervous System Reactions
- Neurotoxic reactions similar to those observed with penicillin G (e.g., lethargy, confusion, twitching, multifocal myoclonus, localized or generalized epileptiform seizures) may occur with large intravenous doses of the penicillinase-resistant penicillins especially in patients with renal insufficiency.
Renal Reactions
- Renal tubular damage and interstitial nephritis have been associated with the administration of methicillin sodium and infrequently with the administration of nafcillin and oxacillin. Manifestations of this reaction may include rash, fever, eosinophilia, hematuria, proteinuria, and renal insufficiency. Methicillin-induced nephropathy does not appear to be dose-related and is generally reversible upon prompt discontinuation of therapy.
Hematologic Reactions
- Eosinophilia, hemolytic anemia, agranulocytosis, neutropenia, leukopenia, granulocytopenia, thrombocytopenia, and bone marrow depression have been associated with the use of penicillinase-resistant penicillins.
Hepatic Reactions
- Hepatotoxicity, characterized by fever, nausea, and vomiting associated with abnormal liver function tests, mainly elevated SGOT levels, has been associated with the use of oxacillin and cloxacillin. Cholestatic hepatitis has been reported rarely. Asymptomatic, transient increases in serum concentrations of alkaline phosphatase, AST(SGOT), and ALT(SGPT) have been reported.
Postmarketing Experience
- Rare reports have been received during postmarketing surveillance of esophageal burning, esophagitis, and esophageal ulceration, particularly after ingestion of dicloxacillin capsules with an insufficient quantity of water and/or before going to bed. To minimize the risk of developing such events, dicloxacillin should be taken with at least 4 fluid ounces (120 mL) of water and dicloxacillin should NOT be taken in the supine position or immediately before going to bed.
Drug Interactions
- Tetracycline, a bacteriostatic antibiotic, may antagonize the bactericidal effect of penicillin and concurrent use of these drugs should be avoided.
- Probenecid administered concomitantly with penicillins increases and prolongs serum penicillin levels.
- Penicillinase-resistant penicillins, like other penicillins, are physically and/or chemically incompatible with aminoglycosides and can inactivate the drugs in vitro. In vitro mixing of penicillins and aminoglycosides should be avoided during concomitant therapy and the drugs should be administered separately. Penicillins can inactivate aminoglycosides in vitro in serum samples from patients receiving both drugs, which could produce falsely decreased results in serum aminoglycoside assays of the serum samples.
- Dicloxacillin may reduce the anticoagulant response to dicumarol and warfarin. Careful monitoring of prothrombin times is suggested during concomitant therapy, and dosage of the anticoagulant should be adjusted as required. The mechanism of this possible interaction is unclear, but may be due to hepatic enzyme induction.
Use in Specific Populations
Pregnancy
- Reproduction studies performed in the mouse, rat, and rabbit have revealed no evidence of impaired fertility or harm to the fetus due to the penicillinase-resistant penicillins. Human experience with the penicillins during pregnancy has not shown any positive evidence of adverse effects on the fetus. There are, however, no adequate or well-controlled studies in pregnant women showing conclusively that harmful effects of these drugs on the fetus can be excluded. Because animal reproduction studies are not always predictive of human response, this drug should be used during pregnancy only if clearly needed.
Pregnancy Category (AUS):
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Dicloxacillin in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Dicloxacillin during labor and delivery.
Nursing Mothers
- Penicillins are excreted in breast milk. Caution should be exercised when penicillins are administered to a nursing woman.
Pediatric Use
- Because of incompletely developed renal function in newborns, penicillinase-resistant penicillins (especially methicillin) may not be completely excreted, with abnormally high blood levels resulting. Frequent blood levels are advisable in this group with dosage adjustments when necessary. All newborns treated with penicillin should be monitored closely for clinical and laboratory evidence of toxic or adverse effects. Experience in the neonatal period is limited. Therefore a dose for the newborn is not recommended.
Geriatic Use
- Clinical studies of dicloxacillin sodium capsules did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects. Other reported clinical experience has not identified differences in responses between the elderly and younger patients. In general, dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy.
Gender
There is no FDA guidance on the use of Dicloxacillin with respect to specific gender populations.
Race
There is no FDA guidance on the use of Dicloxacillin with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Dicloxacillin in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Dicloxacillin in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Dicloxacillin in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Dicloxacillin in patients who are immunocompromised.
Administration and Monitoring
Administration
- Oral
- The penicillinase-resistant penicillins are available for oral administration and for intramuscular and intravenous injection. The sodium salts of methicillin, oxacillin, and nafcillin may be administered parenterally and the sodium salts of cloxacillin, dicloxacillin, oxacillin, and nafcillin are available for oral use.
- Dicloxacillin is best absorbed when taken on an empty stomach, and should be administered at least 1 hour before or 2 hours after meals. Dicloxacillin should be taken with at least 4 fluid ounces (120 mL) of water and should not be taken in the supine position or immediately before going to bed
Monitoring
- Periodic assessment of organ system function including renal, hepatic, and hematopoietic should be made during prolonged therapy with the penicillinase-resistant penicillins.
- Blood cultures, white blood cell, and differential cell counts should be obtained prior to initiation of therapy and at least weekly during therapy with penicillinase-resistant penicillins.
- If any impairment of renal function is suspected or known to exist, a reduction in the total dosage should be considered and blood levels monitored to avoid possible neurotoxic reactions
- AST (SGOT) and ALT (SGPT) values should be obtained periodically during therapy to monitor for possible liver function abnormalities.
IV Compatibility
There is limited information regarding IV Compatibility of Dicloxacillin in the drug label.
Overdosage
There is limited information regarding Overdose of Dicloxacillin in the drug label.
Pharmacology
Mechanism of Action
- Penicillinase-resistant penicillins exert a bactericidal action against penicillin-susceptible microorganisms during the state of active multiplication. All penicillins inhibit the biosynthesis of the bacterial cell wall.
Structure
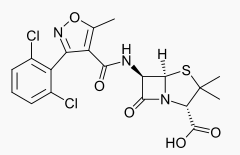
- Dicloxacillin sodium, USP is an antibacterial agent of the isoxazolyl penicillin series. It is a penicillinase-resistant, acid resistant semisynthetic penicillin suitable for oral administration.
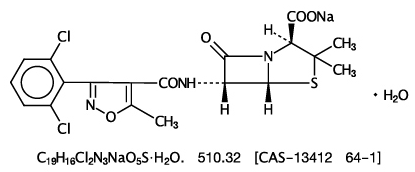
- Dicloxacillin sodium is chemically designated as 4-Thia-1-azabicyclo [3.2.0]heptane-2-carboxylic acid, 6-[3-(2,6-dichlorophenyl)-5-methyl-4-isoxazolyl]-carbonyl]-amino]-3,3-dimethyl-7-oxo-, monosodium salt, monohydrate, [2S-(2α5α,6β)] and has the following structural formula:
Pharmacodynamics
There is limited information regarding Pharmacodynamics of Dicloxacillin in the drug label.
Pharmacokinetics
- Dicloxacillin sodium, USP is resistant to destruction by acid. Absorption of dicloxacillin sodium after oral administration is rapid but incomplete. Food in the gastrointestinal tract decreases the absorption of dicloxacillin. Studies with an oral dose of 125 mg gave average serum levels at 60 minutes of 4.74 mcg/mL. At four hours, average levels were 0.62 mcg/mL. In one study, single oral doses of dicloxacillin sodium 500 mg produced peak serum concentrations of 10 to 17 mcg/mL at 1 to 1.5 hours.
- Once absorbed, dicloxacillin sodium is 95% to 99% bound to serum proteins, mainly albumin.
- Dicloxacillin sodium, with normal doses, has insignificant concentrations in the cerebrospinal and ascitic fluids. It is found in therapeutic concentrations in the pleural, bile, and amniotic fluids. Dicloxacillin sodium is rapidly excreted as unchanged drug in the urine by glomerular filtration and active tubular secretion. The elimination half-life for dicloxacillin sodium is about 0.7 hours.
- Dicloxacillin is not dialyzable. Only minimal amounts are removed by hemodialysis or peritoneal dialysis.
Microbiology
- Dicloxacillin sodium has been shown to be active against most strains of the following microorganisms
Aerobic Gram-Positive Microorganisms
- Staphylococcus spp. (penicillinase producing)
Susceptibility Testing
Diffusion Techniques
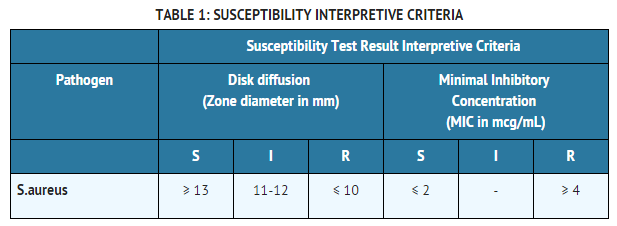
- Quantitative methods that require measurement of zone diameters provide reproducible estimates of the susceptibility of bacteria to antimicrobial compounds. One such standardized procedure1,2 that has been recommended for use with disks to test the susceptibility of microorganisms to dicloxacillin, uses the 1 mcg oxacillin disk. Interpretation involves correlation of the diameter obtained in the disk test with the minimum inhibitory concentration (MIC) for dicloxacillin.
- Reports from the laboratory providing results of the standard single-disk susceptibility test with a 1 mcg oxacillin disk should be interpreted according to the criteria provided in Table 1.
Dilution Techniques
- Quantitative methods that are used to determine minimum inhibitory concentrations (MICs) provide reproducible estimates of the susceptibility of bacteria to antimicrobial compounds. One such standardized procedure1,3 uses a standardized dilution method (broth or agar) or equivalent with oxacillin powder. The MIC values obtained should be interpreted according to the criteria provided in Table 1.
- A report of “Susceptible” (S) indicates that the pathogen is likely to be inhibited by usually achievable concentrations of the antimicrobial compound in the blood. A report of “Intermediate” (I) indicates that the result should be considered equivocal, and, if the microorganism is not fully susceptible to alternative, clinically feasible drugs, the test should be repeated. This category implies possible clinical applicability in body sites where the drug is physiologically concentrated or in situations where high dosage of drug can be used. This category also provides a buffer zone that prevents small uncontrolled technical factors from causing major discrepancies in interpretation. A report of “Resistant” (R) indicates that usually achievable concentrations of the antimicrobial compound in the blood are unlikely to be inhibitory and that other therapy should be selected.
- Measurement of MIC and achieved antimicrobial compound concentrations may be appropriate to guide therapy in some infections. (See CLINICAL PHARMACOLOGY section for further information on drug concentrations achieved in infected body sites and other pharmacokinetic properties of this antimicrobial drug product.)
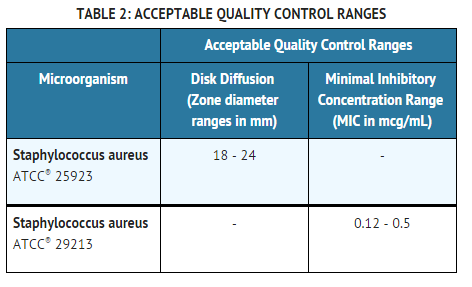
Quality Control
- Standardized susceptibility test procedures require the use of laboratory control microorganisms.The 1 mcg oxacillin disk and the standard oxacillin powder should provide respectively the following zone diameters and MIC values in these laboratory test quality control strains:
Nonclinical Toxicology
Carcinogenesis, Mutagenesis, Impairment of Fertility
- No long-term animal studies have been conducted with these drugs.
- Studies on reproduction (nafcillin) in rats and rabbits reveal no fetal or maternal abnormalities before conception and continuously through weaning (one generation).
Clinical Studies
There is limited information regarding Clinical Studies of Dicloxacillin in the drug label.
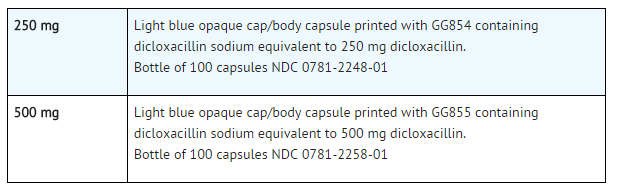
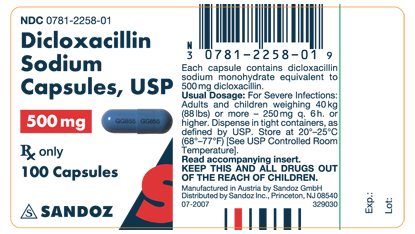
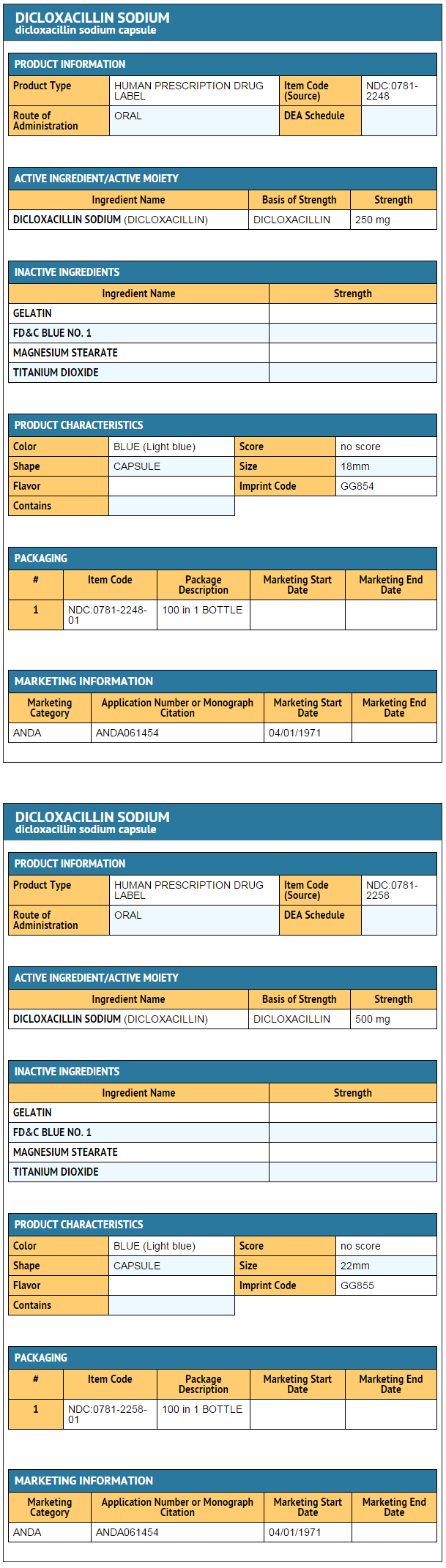
How Supplied
- Dicloxacillin sodium capsules, USP
Storage
- Store at 20°-25°C (68°-77°F)
Images
Drug Images
{{#ask: Page Name::Dicloxacillin |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
250 MG LABEL
NDC 0781-2248-01
Dicloxacillin
Sodium
Capsules, USP
250 mg
Rx only
100 Capsules
SANDOZ
500 MG LABEL
NDC 0781-2258-01
Dicloxacillin
Sodium
Capsules, USP
500 mg
Rx only
100 Capsules
SANDOZ
Ingredients and Appearance
{{#ask: Label Page::Dicloxacillin |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
- Patients receiving penicillins should be given the following information and instructions by the physician:
- Patients should be told that penicillin is an antibacterial agent which will work with the body's natural defenses to control certain types of infections. :* They should be told that the drug should not be taken if they have had an allergic reaction to any form of penicillin previously, and to inform the physician of any allergies or previous allergic reactions to any drugs they may have had.
- Patients who have previously experienced an anaphylactic reaction to penicillin should be instructed to wear a medical identification tag or bracelet.
- Because most antibacterial drugs taken by mouth are best absorbed on an empty stomach, patients should be directed, unless circumstances warrant otherwise, to take penicillin one hour before meals or two hours after eating.
- Patients should be told to take the entire course of therapy prescribed, even if fever and other symptoms have stopped (see PRECAUTIONS – GENERAL).
- If any of the following reactions occur, stop taking your prescription and notify the physician: shortness of breath, wheezing, skin rash, mouth irritation, black tongue, sore throat, nausea, vomiting, diarrhea, fever, swollen joints, or any unusual bleeding or bruising (see ADVERSE REACTIONS).
- Do not take any additional medications without physician approval, including non-prescription drugs such as antacids, laxatives, or vitamins.
- Patients should be counseled that antibacterial drugs including dicloxacillin sodium capsules should only be used to treat bacterial infections. They do not treat viral infections (e.g., the common cold). When dicloxacillin sodium capsules are prescribed to treat a bacterial infection, patients should be told that although it is common to feel better early in the course of therapy, the medication should be taken exactly as directed. Skipping doses or not completing the full course of therapy may: (1) decrease the effectiveness of the immediate treatment, and (2) increase the likelihood that bacteria will develop resistance and will not be treatable by dicloxacillin sodium capsules or other antibacterial drugs in the future.
- Diarrhea is a common problem caused by antibiotics which usually ends when the antibiotic is discontinued. Sometimes after starting treatment with antibiotics, patients can develop watery and bloody stools (with or without stomach cramps and fever) even as late as two or more months after having taken the last dose of the antibiotic. If this occurs, patients should contact their physician as soon as possible.
Precautions with Alcohol
- Alcohol-Dicloxacillin interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
- Dynapen®[3]
Look-Alike Drug Names
There is limited information regarding Dicloxacillin Look-Alike Drug Names in the drug label.
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
- ↑ 1.0 1.1 Stevens DL, Bisno AL, Chambers HF, Dellinger EP, Goldstein EJ, Gorbach SL; et al. (2014). "Practice guidelines for the diagnosis and management of skin and soft tissue infections: 2014 update by the infectious diseases society of America". Clin Infect Dis. 59 (2): 147–59. doi:10.1093/cid/ciu296. PMID 24947530.
- ↑ Kostis J, Bergen SS (1970). "Dicloxacillin therapy of infections by gram-positive organisms". Curr Ther Res Clin Exp. 12 (1): 19–30. PMID 4983478.
- ↑ "Dicloxacillin".