Acetazolamide (extended-release capsule): Difference between revisions
Gerald Chi (talk | contribs) No edit summary |
Gerald Chi- (talk | contribs) No edit summary |
||
| (96 intermediate revisions by 6 users not shown) | |||
| Line 2: | Line 2: | ||
|genericName=acetazolamide | |genericName=acetazolamide | ||
|aOrAn=a | |aOrAn=a | ||
|drugClass=carbonic anhydrase inhibitor | |drugClass=[[carbonic anhydrase]] [[inhibitor]] | ||
|indicationType=treatment | |||
|indication=chronic [[glaucoma|primary open-angle glaucoma]] and [[glaucoma|secondary glaucoma]]. Acetazolamide is also indicated for the prevention or amelioration of symptoms associated with acute [[mountain sickness]] despite gradual ascent | |indication=chronic [[glaucoma|primary open-angle glaucoma]] and [[glaucoma|secondary glaucoma]]. Acetazolamide is also indicated for the prevention or amelioration of symptoms associated with acute [[mountain sickness]] despite gradual ascent | ||
|adverseReactions=[[dizziness]] and [[lightheadedness]] | |adverseReactions=[[dizziness]] and [[lightheadedness]] | ||
|blackBoxWarningTitle= | |blackBoxWarningTitle=<b><span style="color:#FF0000;">TITLE</span></b> | ||
|blackBoxWarningBody=<i><span style="color:#FF0000;">Condition Name:</span></i> (Content) | |blackBoxWarningBody=<i><span style="color:#FF0000;">Condition Name:</span></i> (Content) | ||
|fdaLIADAdult======Glaucoma===== | |fdaLIADAdult======Glaucoma===== | ||
| Line 11: | Line 12: | ||
* Dosing Information | * Dosing Information | ||
:* The recommended dosage is 1 capsule (500 mg) two times a day. Usually 1 capsule is administered in the morning and 1 capsule in the evening | :* The recommended dosage is '''1 capsule (500 mg) two times a day'''. Usually 1 capsule is administered in the morning and 1 capsule in the evening. | ||
In those unusual instances where adequate control is not obtained by the twice-a-day administration of | :* In those unusual instances where adequate control is not obtained by the twice-a-day administration of [[acetazolamide]], the desired control may be established by means of [[acetazolamide]] (tablets or parenteral). Use tablets or parenteral in accordance with the more frequent dosage schedules recommended for these dosage forms, such as '''250 mg every four hours''', or an initial dose of '''500 mg followed by 250 mg or 125 mg every four hours''', depending on the case in question. | ||
=====Acute Mountain Sickness===== | =====Acute Mountain Sickness===== | ||
| Line 18: | Line 19: | ||
* Dosing Information | * Dosing Information | ||
:* | :* The recommended dosage is '''500 mg to 1000 mg daily''', in divided doses using tablets or extended-release capsules as appropriate. In circumstances of rapid ascent, such as in rescue or military operations, the higher dose level of '''1000 mg''' is recommended. It is preferable to initiate dosing 24 to 48 hours before ascent and to continue for 48 hours while at high altitude, or longer as necessary to control symptoms. | ||
|offLabelAdultGuideSupport= | |offLabelAdultGuideSupport=There is limited information regarding <i>Off-Label Guideline-Supported Use</i> of [[acetazolamide]] in adult patients. | ||
|offLabelAdultNoGuideSupport======Glaucoma Following Surgery===== | |||
|offLabelAdultNoGuideSupport====== | |||
* Dosing Information | * Dosing Information | ||
:* | :* '''500 mg PO 1 hour postoperatively'''<ref name="Byrd-1998">{{Cite journal | last1 = Byrd | first1 = S. | last2 = Singh | first2 = K. | title = Medical control of intraocular pressure after cataract surgery. | journal = J Cataract Refract Surg | volume = 24 | issue = 11 | pages = 1493-7 | month = Nov | year = 1998 | doi = | PMID = 9818340 }}</ref> | ||
===== | |||
=====Macular Retinal Edema===== | |||
* Dosing Information | * Dosing Information | ||
:* | :* '''500 mg PO qd for 4 weeks'''<ref name="Whitcup-1996">{{Cite journal | last1 = Whitcup | first1 = SM. | last2 = Csaky | first2 = KG. | last3 = Podgor | first3 = MJ. | last4 = Chew | first4 = EY. | last5 = Perry | first5 = CH. | last6 = Nussenblatt | first6 = RB. | title = A randomized, masked, cross-over trial of [[acetazolamide]] for cystoid macular edema in patients with uveitis. | journal = Ophthalmology | volume = 103 | issue = 7 | pages = 1054-62; discussion 1062-3 | month = Jul | year = 1996 | doi = | PMID = 8684794 }}</ref> | ||
===== | =====Periodic Ataxia===== | ||
* Dosing Information | * Dosing Information | ||
:* | :* Doses of '''250 to 1000 mg/day''' have eliminated or decreased the frequency and severity of symptoms such as [[nausea]], [[vomiting]], [[diplopia]], and [[nystagmus]].<ref name="Baloh-1991">{{Cite journal | last1 = Baloh | first1 = RW. | last2 = Winder | first2 = A. | title = [[acetazolamide]]-responsive vestibulocerebellar syndrome: clinical and oculographic features. | journal = Neurology | volume = 41 | issue = 3 | pages = 429-33 | month = Mar | year = 1991 | doi = | PMID = 2006014 }}</ref><ref name="Friedman-1987">{{Cite journal | last1 = Friedman | first1 = JH. | last2 = Hollmann | first2 = PA. | title = [[acetazolamide]] responsive hereditary paroxysmal ataxia. | journal = Mov Disord | volume = 2 | issue = 1 | pages = 67-72 | month = | year = 1987 | doi = 10.1002/mds.870020110 | PMID = 3332806 }}</ref><ref name="Bouchard-1984">{{Cite journal | last1 = Bouchard | first1 = JP. | last2 = Roberge | first2 = C. | last3 = van Gelder | first3 = NM. | last4 = Barbeau | first4 = A. | title = Familial periodic ataxia responsive to [[acetazolamide]]. | journal = Can J Neurol Sci | volume = 11 | issue = 4 Suppl | pages = 550-3 | month = Nov | year = 1984 | doi = | PMID = 6439406 }}</ref><ref name="Griggs-1978">{{Cite journal | last1 = Griggs | first1 = RC. | last2 = Moxley | first2 = RT. | last3 = Lafrance | first3 = RA. | last4 = McQuillen | first4 = J. | title = Hereditary paroxysmal ataxia: response to [[acetazolamide]]. | journal = Neurology | volume = 28 | issue = 12 | pages = 1259-64 | month = Dec | year = 1978 | doi = | PMID = 366453 }}</ref> | ||
| | |fdaLIADPed=The safety and effectiveness of [[acetazolamide]] in pediatric patients below the age of 12 years have not been established. [[Growth retardation]] has been reported in children receiving long-term therapy, believed secondary to chronic [[acidosis]]. | ||
|offLabelPedGuideSupport=There is limited information regarding <i>Off-Label Guideline-Supported Use</i> of [[acetazolamide]] in pediatric patients. | |||
|offLabelPedNoGuideSupport=There is limited information regarding <i>Off-Label Non–Guideline-Supported Use</i> of [[acetazolamide]] in pediatric patients. | |||
|contraindications=* [[Hypersensitivity]] to [[acetazolamide]] or any excipients in the formulation. | |||
:* Since [[acetazolamide]] is a [[sulfonamide]] derivative, cross sensitivity between [[acetazolamide]], [[sulfonamide]]s and other [[sulfonamide]] derivatives is possible. | |||
: | |||
===== | |||
* | * [[Hyponatremia]] and [[hypokalemia]] | ||
:* [[Acetazolamide]] therapy is contraindicated in situations in which [[sodium]] and/or [[potassium]] blood serum levels are depressed, in cases of marked kidney and liver disease or dysfunction, in [[adrenal insufficiency|suprarenal gland failure]], and in [[hyperchloremic acidosis]]. | |||
* | * [[Cirrhosis]] | ||
:* [[Acetazolamide]] is contraindicated in patients with [[cirrhosis]] because of the risk of development of [[hepatic encephalopathy]]. | |||
* | * Chronic non-congestive angle-closure [[glaucoma]] | ||
:* Long-term administration of [[acetazolamide]] is contraindicated in patients with chronic non-congestive angle-closure [[glaucoma]] since it may permit organic closure of the angle to occur while the worsening [[glaucoma]] is masked by lowered [[intraocular pressure]]. | |||
|warnings=* Fatalities have occurred, although rarely, due to severe reactions to [[sulfonamide]]s including [[Stevens-Johnson syndrome]], [[toxic epidermal necrolysis]], [[fulminant hepatic necrosis]], [[anaphylaxis]], [[agranulocytosis]], [[aplastic anemia]], and other blood [[dyscrasia]]s. | |||
* | * Sensitizations may recur when a [[sulfonamide]] is readministered irrespective of the route of administration. If signs of [[hypersensitivity]] or other serious reactions occur, discontinue use of this drug. | ||
* Caution is advised for patients receiving concomitant high-dose [[aspirin]] and [[acetazolamide]], as [[anorexia]], [[tachypnea]], [[lethargy]], [[metabolic acidosis]], [[coma]], and [[death]] have been reported. | |||
* | * Increasing the dose does not increase the [[diuresis]] and may increase the incidence of [[drowsiness]] and/or [[paresthesia]]. Increasing the dose often results in a decrease in [[diuresis]]. Under certain circumstances, however, very large doses have been given in conjunction with other [[diuretic]]s in order to secure [[diuresis]] in complete refractory failure. | ||
* To monitor for hematologic reactions common to all [[sulfonamide]]s, it is recommended that a baseline [[CBC]] and [[platelet]] count be obtained on patients prior to initiating [[acetazolamide]] therapy and at regular intervals during therapy. If significant changes occur, early discontinuance and institution of appropriate therapy are important. Periodic monitoring of serum [[electrolyte]]s is recommended. | |||
|clinicalTrials=There is limited information regarding <i>Clinical Trial Experience</i> of [[acetazolamide]] in the drug label. | |||
|postmarketing====Body as a whole=== | |||
[[Headache]], [[malaise]], [[fatigue]], [[fever]], [[pain]] at injection site, [[flushing]], [[growth retardation]] in children, [[flaccid paralysis]], [[anaphylaxis]]. | |||
[[Headache]], [[malaise]], [[fatigue]], fever, pain at injection site, [[flushing]], growth retardation in children, [[flaccid paralysis]], [[anaphylaxis]]. | |||
===Digestive=== | ===Digestive=== | ||
| Line 131: | Line 72: | ||
===Hematological/Lymphatic=== | ===Hematological/Lymphatic=== | ||
Blood | Blood [[dyscrasia]]s such as [[aplastic anemia]], [[agranulocytosis]], [[leukopenia]], [[thrombocytopenic purpura]], [[melena]]. | ||
===Hepato-biliary disorders=== | ===Hepato-biliary disorders=== | ||
Abnormal liver function, [[cholestatic jaundice]], [[hepatic insufficiency]], fulminant hepatic necrosis. | Abnormal [[liver function test|liver function]], [[cholestatic jaundice]], [[hepatic insufficiency]], [[fulminant hepatic necrosis]]. | ||
===Metabolic/Nutritional=== | ===Metabolic/Nutritional=== | ||
Metabolic acidosis, electrolyte imbalance, including [[hypokalemia]], [[hyponatremia]], [[osteomalacia]] with long-term phenytoin therapy, loss of appetite, taste alteration, hyper/[[hypoglycemia]]. | [[Metabolic acidosis]], [[electrolyte imbalance]], including [[hypokalemia]], [[hyponatremia]], [[osteomalacia]] with long-term [[phenytoin]] therapy, loss of [[appetite]], taste alteration, [[hyperglycemia|hyper]]/[[hypoglycemia]]. | ||
===Nervous=== | ===Nervous=== | ||
| Line 147: | Line 88: | ||
===Skin=== | ===Skin=== | ||
Allergic skin reactions including [[urticaria]], [[photosensitivity]], [[Stevens- Johnson syndrome]], [[toxic epidermal necrolysis]]. | [[Allergic]] skin reactions including [[urticaria]], [[photosensitivity]], [[Stevens-Johnson syndrome]], [[toxic epidermal necrolysis]]. | ||
===Special senses=== | ===Special senses=== | ||
Hearing disturbances, [[tinnitus]], transient [[myopia]]. | Hearing disturbances, [[tinnitus]], transient [[myopia]]. | ||
|drugInteractions=* [[Aspirin]] | |||
|drugInteractions=* Aspirin | :* Caution is advised for patients receiving concomitant high-dose [[aspirin]] and [[acetazolamide]], as [[anorexia]], [[tachypnea]], [[lethargy]], [[metabolic acidosis]], [[coma]], and [[death]] have been reported. | ||
:* | * [[Anticonvulsants]] | ||
Because of possible additive effects with other carbonic anhydrase inhibitors, concomitant use is not advisable. | :* [[Acetazolamide]] modifies [[phenytoin]] metabolism with increased serum levels of [[phenytoin]]. This may increase or enhance the occurrence of [[osteomalacia]] in some patients receiving chronic [[phenytoin]] therapy. Caution is advised in patients receiving chronic concomitant therapy. By decreasing the gastrointestinal absorption of [[primidone]], [[acetazolamide]] may decrease serum concentrations of [[primidone]] and its metabolites, with a consequent possible decrease in [[anticonvulsant]] effect. Caution is advised when beginning, discontinuing, or changing the dose of [[acetazolamide]] in patients receiving [[primidone]]. | ||
Acetazolamide may increase the effects of other folic acid | * [[Carbonic anhydrase]] inhibitors | ||
Acetazolamide decreases urinary excretion of amphetamine and may enhance the magnitude and duration of their effect. | :* Because of possible additive effects with other [[carbonic anhydrase]] inhibitors, concomitant use is not advisable. | ||
Acetazolamide reduces urinary excretion of quinidine and may enhance its effect. | * [[Folic acid]] antagonists | ||
Acetazolamide may prevent the urinary antiseptic effect of methenamine. Acetazolamide increases lithium excretion and the lithium may be decreased. | :* [[Acetazolamide]] may increase the effects of other [[folic acid]] [[antagonist]]s. | ||
Acetazolamide and sodium bicarbonate used concurrently increase the risk of renal calculus formation. | * [[Amphetamine]] | ||
Acetazolamide may elevate cyclosporine levels. | :* [[Acetazolamide]] decreases urinary excretion of [[amphetamine]] and may enhance the magnitude and duration of their effect. | ||
* [[Quinidine]] | |||
:* [[Acetazolamide]] reduces urinary excretion of [[quinidine]] and may enhance its effect. | |||
* [[Methenamine]] | |||
:* [[Acetazolamide]] may prevent the urinary antiseptic effect of [[methenamine]]. | |||
* [[Lithium]] | |||
:* [[Acetazolamide]] increases [[lithium]] excretion and the lithium may be decreased. | |||
* [[Sodium bicarbonate]] | |||
:* [[Acetazolamide]] and [[sodium bicarbonate]] used concurrently increase the risk of renal [[calculus]] formation. | |||
* [[Cyclosporine]] | |||
:* [[Acetazolamide]] may elevate [[cyclosporine]] levels. | |||
* Drug/laboratory test interactions | * Drug/laboratory test interactions | ||
:* | :* [[Sulfonamide]]s may give false negative or decreased values for urinary phenolsulfonphthalein and phenol red elimination values for urinary protein, serum non-protein, and serum [[uric acid]]. | ||
Acetazolamide interferes with the HPLC method of assay for theophylline. Interference with the theophylline assay by acetazolamide depends on the solvent used in the extraction; acetazolamide may not interfere with other assay methods for theophylline. | :* [[Acetazolamide]] may produce an increased level of crystals in the urine. | ||
:* [[Acetazolamide]] interferes with the HPLC method of assay for [[theophylline]]. Interference with the [[theophylline]] assay by [[acetazolamide]] depends on the solvent used in the extraction; [[acetazolamide]] may not interfere with other assay methods for [[theophylline]]. | |||
|FDAPregCat=C | |FDAPregCat=C | ||
|useInPregnancyFDA======Teratogenic effects | |useInPregnancyFDA======Teratogenic effects===== | ||
[[acetazolamide]], administered orally or parenterally, has been shown to be teratogenic (defects of the limbs) in mice, rats, hamsters, and rabbits. There are no adequate and well-controlled studies in pregnant women. [[Acetazolamide]] should be used in [[pregnancy]] only if the potential benefit justifies the potential risk to the fetus. | |||
|useInNursing=Because of the potential for serious adverse reactions in nursing infants from | |AUSPregCat=B3 | ||
|useInPed=The safety and effectiveness of | |useInNursing=Because of the potential for serious adverse reactions in nursing infants from [[acetazolamide]], a decision should be made whether to discontinue nursing or to discontinue the drug taking into account the importance of the drug to the mother. [[Acetazolamide]] should only be used by nursing women if the potential benefit justifies the potential risk to the child. | ||
|useInGeri=Metabolic acidosis, which can be severe, may occur in the elderly with reduced renal function. | |useInPed=The safety and effectiveness of [[acetazolamide]] in pediatric patients below the age of 12 years have not been established. [[Growth retardation]] has been reported in children receiving long-term therapy, believed secondary to chronic [[acidosis]]. | ||
|useInGeri=Metabolic acidosis, which can be severe, may occur in the elderly with reduced [[renal function]]. | |||
In general, dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and concomitant disease or other drug therapy. | In general, dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and concomitant disease or other drug therapy. | ||
|administration=Oral | |||
|monitoring=======Electrolyte Imbalances====== | |||
|administration= | |||
|monitoring====== | |||
[[Acetazolamide]] treatment may cause [[electrolyte imbalance]]s, including [[hyponatremia]] and [[hypokalemia]], as well as [[metabolic acidosis]]. Therefore, periodic monitoring of serum [[electrolyte]]s is recommended. | |||
======Hematologic Distrubances====== | |||
To monitor for hematologic reactions common to all [[sulfonamide]]s, it is recommended that a baseline [[CBC]] and [[platelet]] count be obtained on patients prior to initiating [[acetazolamide]] therapy and at regular intervals during therapy. If significant changes occur, early discontinuance and institution of appropriate therapy are important. | |||
|IVCompat=There is limited information regarding <i>IV Compatibility</i> of [[acetazolamide]] in the drug label. | |||
|IVCompat= | |||
|overdose====Acute Overdose=== | |overdose====Acute Overdose=== | ||
====Management==== | ====Management==== | ||
* No specific antidote is known. | |||
( | * Treatment should be symptomatic and supportive. [[Electrolyte imbalance]], development of an [[acidosis|acidotic]] state, and central nervous system effects might be expected to occur. Serum [[electrolyte]] levels (particularly [[potassium]]) and blood pH levels should be monitored. Supportive measures are required to restore [[electrolyte]] and pH balance. The acidotic state can usually be corrected by the administration of [[bicarbonate]]. | ||
* Despite its high intraerythrocytic distribution and plasma protein binding properties, [[acetazolamide]] may be dialyzable. This may be particularly important in the management of [[acetazolamide]] overdosage when complicated by the presence of [[renal failure]]. | |||
|drugBox={{Drugbox2 | |drugBox={{Drugbox2 | ||
| verifiedrevid = | | verifiedrevid = 477238754 | ||
| IUPAC_name = | | IUPAC_name = ''N''-(5-sulfamoyl-1,3,4-thiadiazol-2-yl)acetamide | ||
| image = | | image = Acetazolamide2DACS.svg.png | ||
| | | width = 200px | ||
| image2 = Acetazolamide3Dan.gif | |||
| width2 = 200px | |||
<!--Clinical data--> | <!--Clinical data--> | ||
| tradename = | | tradename = [[acetazolamide]] | ||
| | | pregnancy_AU = B3 | ||
| | | pregnancy_US = C | ||
| | | legal_AU = S4 | ||
| | | legal_CA = Rx-only | ||
| | | legal_UK = POM | ||
| routes_of_administration = | | legal_US = Rx-only | ||
| routes_of_administration = oral or [[intravenous]] | |||
<!--Pharmacokinetic data--> | <!--Pharmacokinetic data--> | ||
| bioavailability = | | bioavailability = | ||
| metabolism = | | protein_binding = 70-90% | ||
| elimination_half-life = | | metabolism = None | ||
| excretion = | | elimination_half-life = 2-4 hours | ||
| excretion = Urine (90%) | |||
<!--Identifiers--> | <!--Identifiers--> | ||
| CAS_number_Ref = | | CASNo_Ref = {{cascite|correct|CAS}} | ||
| CAS_number = | | CAS_number_Ref = {{cascite|correct|??}} | ||
| ATC_prefix = | | CAS_number = 59-66-5 | ||
| ATC_suffix = | | ATC_prefix = S01 | ||
| PubChem = | | ATC_suffix = EC01 | ||
| | | PubChem = 1986 | ||
| | | DrugBank_Ref = {{drugbankcite|correct|drugbank}} | ||
| DrugBank = | | DrugBank = DB00819 | ||
| ChemSpiderID_Ref = | | ChemSpiderID_Ref = {{chemspidercite|correct|chemspider}} | ||
| ChemSpiderID = | | ChemSpiderID = 1909 | ||
| UNII_Ref = | | UNII_Ref = {{fdacite|correct|FDA}} | ||
| UNII = | | UNII = O3FX965V0I | ||
| KEGG_Ref = | | KEGG_Ref = {{keggcite|correct|kegg}} | ||
| KEGG = | | KEGG = D00218 | ||
| ChEBI_Ref = | | ChEBI_Ref = {{ebicite|correct|EBI}} | ||
| ChEBI = | | ChEBI = 27690 | ||
| ChEMBL_Ref = | | ChEMBL_Ref = {{ebicite|correct|EBI}} | ||
| ChEMBL = | | ChEMBL = 20 | ||
| PDB_ligand = AZM | |||
<!--Chemical data--> | <!--Chemical data--> | ||
| C= | H= | N= | O= | | C=4 | H=6 | N=4 | O=3 | S=2 | ||
| molecular_weight = | | molecular_weight = 222.245 g/mol | ||
| smiles = | | smiles = O=S(=O)(c1nnc(s1)NC(=O)C)N | ||
| InChI = | | InChI = 1/C4H6N4O3S2/c1-2(9)6-3-7-8-4(12-3)13(5,10)11/h1H3,(H2,5,10,11)(H,6,7,9) | ||
| | | StdInChI_Ref = {{stdinchicite|correct|chemspider}} | ||
| | | StdInChI = 1S/C4H6N4O3S2/c1-2(9)6-3-7-8-4(12-3)13(5,10)11/h1H3,(H2,5,10,11)(H,6,7,9) | ||
| StdInChI = | | StdInChIKey_Ref = {{stdinchicite|correct|chemspider}} | ||
| StdInChIKey_Ref = | | StdInChIKey = BZKPWHYZMXOIDC-UHFFFAOYSA-N | ||
| StdInChIKey = | |||
}} | }} | ||
|mechAction=( | |mechAction=* [[Acetazolamide]] is a potent [[carbonic anhydrase]] [[inhibitor]], effective in the control of fluid secretion (e.g., some types of [[glaucoma]]), in the treatment of certain convulsive disorders (e.g., [[epilepsy]]), and in the promotion of [[diuresis]] in instances of abnormal fluid retention (e.g., cardiac [[edema]]). | ||
* [[Acetazolamide]] is not a mercurial [[diuretic]]. Rather, it is a non-[[bacteriostatic]] [[sulfonamide]] possessing a chemical structure and pharmacological activity distinctly different from the [[bacteriostatic]] [[sulfonamide]]s. | |||
( | * [[Acetazolamide]] is an [[enzyme]] [[inhibitor]] that acts specifically on [[carbonic anhydrase]], the enzyme that catalyzes the reversible reaction involving the hydration of [[carbon dioxide]] and the dehydration of carbonic acid. In the eye, this inhibitory action of [[acetazolamide]] decreases the secretion of [[aqueous humor]] and results in a drop in [[intraocular pressure]], a reaction considered desirable in cases of [[glaucoma]] and even in certain non-glaucomatous conditions. Evidence seems to indicate that [[acetazolamide]] has utility as an adjuvant in treatment of certain dysfunctions of the [[central nervous system]] (e.g., [[epilepsy]]). Inhibition of [[carbonic anhydrase]] in this area appears to retard abnormal, paroxysmal, excessive discharge from [[central nervous system]] [[neuron]]s. The [[diuretic]] effect of [[acetazolamide]] is due to its action in the kidney on the reversible reaction involving hydration of [[carbon dioxide]] and dehydration of carbonic acid. The result is renal loss of [[bicarbonate|HCO3]] ion, which carries out [[sodium]], water, and [[potassium]]. Alkalinization of the urine and promotion of [[diuresis]] are thus affected. Alteration in [[ammonia]] metabolism occurs due to increased reabsorption of [[ammonia]] by the [[renal tubule]]s as a result of urinary alkalinization. | ||
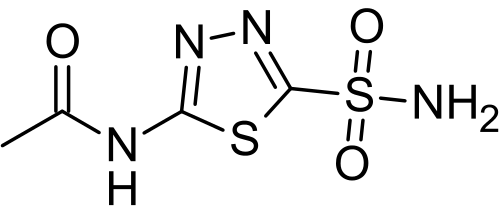
|structure=The chemical name for acetazolamide is N-(5-Sulfamoyl-1,3, 4-thiadiazol-2-yl) acetamide and has the following chemical structure: | |||
== | [[File:Acetazolamide001.jpeg|400px|thumb|none|This image is provided by the National Library of Medicine.]] | ||
|PD=* [[Acetazolamide]] provide prolonged action to inhibit aqueous humor secretion for 18 to 24 hours after each dose, whereas tablets act for only eight to 12 hours. The prolonged continuous effect of [[acetazolamide]] permits a reduction in dosage frequency. | |||
|PK=* Plasma concentrations of acetazolamide peak from three to six hours after administration of [[acetazolamide]], compared to one to four hours with tablets. Food does not affect bioavailability of [[acetazolamide]]. | |||
( | * [[Placebo]]-controlled clinical trials have shown that prophylactic administration of [[acetazolamide]] at a dose of 250 mg every eight to 12 hours (or a 500 mg controlled release [[capsule]] once daily) before and during rapid ascent to altitude results in fewer and/or less severe symptoms of acute [[mountain sickness]] (AMS) such as [[headache]], [[nausea]], [[shortness of breath]], [[dizziness]], [[drowsiness]], and [[fatigue]]. Pulmonary function (e.g., [[minute ventilation]], expired vital capacity, and peak flow) is greater in the [[acetazolamide]] treated group, both in subjects with AMS and asymptomatic subjects. The [[acetazolamide]] treated climbers also had less difficulty in sleeping. | ||
|nonClinToxic=There is limited information regarding <i>Nonclinical Toxicology</i> of [[acetazolamide]] in the drug label. | |||
|clinicalStudies=There is limited information regarding <i>Clinical Studies</i> of acetazolamide in the drug label. | |||
|howSupplied=* Diamox Sequels ([[Acetazolamide]] Extended-Release Capsules) are available as 500 mg: | |||
: Orange opaque cap and orange opaque body filled with white to off-white pellets. Imprinted in black ink, DIAMOX 754. | |||
== | * Available in bottles of: | ||
: 100 NDC 51285-754-02 | |||
: DURAMED PHARMACEUTICALS, INC. | |||
: Subsidiary of Barr Pharmaceuticals, Inc. | |||
: Pomona, New York 10970 | |||
: Revised JULY 2008 | |||
: BR-754 | |||
|storage=Store at 20° to 25°C (68° to 77°F). | |||
|packLabel=[[File:DIAMOX SEQUELS (acetazolamide) capsule, extended release label display.jpeg|thumb|none|400px|This image is provided by the National Library of Medicine.]] | |||
[[File:Acetazolamide004.jpg|thumb|none|400px|This image is provided by the National Library of Medicine.]] | |||
|fdaPatientInfo=* [[Adverse reaction]]s common to all [[sulfonamide]] derivatives may occur: [[anaphylaxis]], [[fever]], [[rash]] (including [[erythema multiforme]], [[Stevens-Johnson syndrome]], [[toxic epidermal necrolysis]]), [[crystalluria]], [[renal]] [[calculus]], [[myelosuppression|bone marrow depression]], [[thrombocytopenic purpura]], [[hemolytic anemia]], [[leukopenia]], [[pancytopenia]], and [[agranulocytosis]]. Caution is advised for early detection of such reactions and the drug should be discontinued and appropriate therapy instituted. | |||
|fdaPatientInfo=Adverse | * In patients with pulmonary obstruction or [[emphysema]] where alveolar [[ventilation]] may be impaired, [[acetazolamide]] which may precipitate or aggravate [[acidosis]] should be used with caution. | ||
In patients with pulmonary obstruction or emphysema where alveolar ventilation may be impaired, | |||
Gradual ascent is desirable to try to avoid acute mountain sickness. If rapid ascent is undertaken and | * Gradual ascent is desirable to try to avoid acute [[mountain sickness]]. If rapid ascent is undertaken and [[acetazolamide]] is used, it should be noted that such use does not obviate the need for prompt descent if severe forms of high altitude sickness occur, i.e., high altitude pulmonary edema (HAPE) or high altitude [[cerebral edema]]. | ||
Caution is advised for patients receiving concomitant high-dose aspirin and | |||
Both increases and decreases in blood glucose have been described in patients treated with acetazolamide. This should be taken into consideration in patients with impaired glucose tolerance or diabetes mellitus. | * Caution is advised for patients receiving concomitant high-dose [[aspirin]] and [[acetazolamide]], as [[anorexia]], [[tachypnea]], [[lethargy]], [[metabolic acidosis]], [[coma]], and [[death]] have been reported. | ||
Acetazolamide treatment may cause electrolyte | |||
Some adverse reactions to acetazolamide, such as drowsiness, fatigue, and myopia, may impair the ability to drive and operate machinery. | * Both increases and decreases in blood [[glucose]] have been described in patients treated with [[acetazolamide]]. This should be taken into consideration in patients with [[impaired glucose tolerance]] or [[diabetes mellitus]]. | ||
|alcohol=Alcohol- | |||
|lookAlike=* | * [[Acetazolamide]] treatment may cause [[electrolyte imbalance]]s, including [[hyponatremia]] and [[hypokalemia]], as well as [[metabolic acidosis]]. Therefore, periodic monitoring of [[serum electrolyte]]s is recommended. Particular caution is recommended in patients with conditions that are associated with, or predispose a patient to, [[electrolyte]] and [[Acid-base imbalance|acid/base imbalances]], such as patients with impaired [[renal function]] (including elderly patients), patients with [[diabetes mellitus]], and patients with impaired [[alveolar]] [[ventilation]]. | ||
* | |||
* Some adverse reactions to [[acetazolamide]], such as [[drowsiness]], [[fatigue]], and [[myopia]], may impair the ability to drive and operate machinery.<ref name="dailymed.nlm.nih.gov">{{Cite web | last = | first = | title = DIAMOX SEQUELS (ACETAZOLAMIDE) CAPSULE, EXTENDED RELEASE [DURAMED PHARMACEUTICALS, INC.] | url = http://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=74e47451-2bc8-496e-88ad-c10002ee8e22 | publisher = | date = | accessdate = }}</ref> | |||
|alcohol=Alcohol-[[acetazolamide]] interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication. | |||
|brandNames=Diamox Sequels® | |||
|lookAlike=* acetaZOLAMIDE — acetoHEXAMIDE<ref name="www.ismp.org">{{Cite web | last = | first = | title = http://www.ismp.org | url = http://www.ismp.org | publisher = | date = }}</ref> | |||
* Diamox® — Diabinese®<ref name="www.ismp.org">{{Cite web | last = | first = | title = http://www.ismp.org | url = http://www.ismp.org | publisher = | date = }}</ref> | |||
|nlmPatientInfo=(Link to patient information page) | |nlmPatientInfo=(Link to patient information page) | ||
|drugShortage= | |drugShortage= | ||
}} | |||
{{PillImage | |||
|fileName=Diamox_Sequels_NDC_512850754.jpg | |||
|drugName=Diamox Sequels | |||
|NDC=512850754 | |||
|drugAuthor=Duramed Pharmaceuticals, Inc. | |||
|ingredients=ACETAZOLAMIDE[ACETAZOLAMIDE] | |||
|pillImprint=DIAMOX;754 | |||
|dosageValue=500 | |||
|dosageUnit=mg | |||
|pillColor=Orange | |||
|pillShape=Capsule | |||
|pillSize=23 | |||
|pillScore=1 | |||
}} | }} | ||
{{PillImage | |||
|fileName=Acetazolamide_NDC_516724022.jpg | |||
|drugName=Acetazolamide | |||
|NDC=516724022 | |||
|drugAuthor=Taro Pharmaceuticals U.S.A., Inc. | |||
|ingredients=Acetazolamide[Acetazolamide] | |||
|pillImprint=T52 | |||
|dosageValue=125 | |||
|dosageUnit=mg | |||
|pillColor=White | |||
|pillShape=Round | |||
|pillSize=9 | |||
|pillScore=2 | |||
}} | |||
{{PillImage | |||
|fileName=Acetazolamide_NDC_516724023.jpg | |||
|drugName=Acetazolamide | |||
|NDC=516724023 | |||
|drugAuthor=Taro Pharmaceuticals U.S.A., Inc. | |||
|ingredients=Acetazolamide[Acetazolamide] | |||
|pillImprint=T53 | |||
|dosageValue=250 | |||
|dosageUnit=mg | |||
|pillColor=White | |||
|pillShape=Round | |||
|pillSize=11 | |||
|pillScore=4 | |||
}} | |||
{{PillImage | |||
|fileName=AcetaZOLAMIDE_NDC_604290033.jpg | |||
|drugName=AcetaZOLAMIDE | |||
|NDC=604290033 | |||
|drugAuthor=Golden State Medical Supply, Inc. | |||
|ingredients=Acetazolamide[Acetazolamide] | |||
|pillImprint=T52 | |||
|dosageValue=125 | |||
|dosageUnit=mg | |||
|pillColor=White | |||
|pillShape=Round | |||
|pillSize=9 | |||
|pillScore=2 | |||
}} | |||
{{PillImage | |||
|fileName=AcetaZOLAMIDE_NDC_604290034.jpg | |||
|drugName=AcetaZOLAMIDE | |||
|NDC=604290034 | |||
|drugAuthor=Golden State Medical Supply, Inc. | |||
|ingredients=Acetazolamide[Acetazolamide] | |||
|pillImprint=T53 | |||
|dosageValue=250 | |||
|dosageUnit=mg | |||
|pillColor=White | |||
|pillShape=Round | |||
|pillSize=11 | |||
|pillScore=4 | |||
}} | |||
{{PillImage | |||
|fileName=AcetaZOLAMIDE_NDC_05271050.jpg | |||
|drugName=AcetaZOLAMIDE | |||
|NDC=05271050 | |||
|drugAuthor=Lannett Company, Inc. | |||
|ingredients=ACETAZOLAMIDE[ACETAZOLAMIDE] | |||
|pillImprint=LAN;1050 | |||
|dosageValue=250 | |||
|dosageUnit=mg | |||
|pillColor=White | |||
|pillShape=Round | |||
|pillSize=11 | |||
|pillScore=4 | |||
}} | |||
{{PillImage | |||
|fileName=Acetazolamide_NDC_05550513.jpg | |||
|drugName=Acetazolamide | |||
|NDC=05550513 | |||
|drugAuthor=Barr Laboratories Inc.. | |||
|ingredients=ACETAZOLAMIDE[ACETAZOLAMIDE] | |||
|pillImprint=barr;513 | |||
|dosageValue=500 | |||
|dosageUnit=mg | |||
|pillColor=Orange | |||
|pillShape=Capsule | |||
|pillSize=23 | |||
|pillScore=1 | |||
}} | |||
{{PillImage | |||
|fileName=Acetazolamide_NDC_683820261.jpg | |||
|drugName=Acetazolamide | |||
|NDC=683820261 | |||
|drugAuthor=Zydus Pharmaceuticals (USA) Inc. | |||
|ingredients=ACETAZOLAMIDE[ACETAZOLAMIDE] | |||
|pillImprint=EP;107 | |||
|dosageValue=500 | |||
|dosageUnit=mg | |||
|pillShape=Capsule | |||
|pillSize=25 | |||
|pillScore=1 | |||
}} | |||
{{LabelImage | |||
|fileName=DIAMOX SEQUELS (acetazolamide) capsule, extended release label display.jpeg | |||
}} | |||
{{LabelImage | |||
|fileName=Acetazolamide004.jpg | |||
}} | |||
[[Category: Cardiovascular Drugs]] | |||
[[Category: Diuretics]] | |||
[[Category: Drug]] | |||
Latest revision as of 22:16, 14 May 2015
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1];
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Acetazolamide (extended-release capsule) is a carbonic anhydrase inhibitor that is FDA approved for the treatment of chronic primary open-angle glaucoma and secondary glaucoma. Acetazolamide is also indicated for the prevention or amelioration of symptoms associated with acute mountain sickness despite gradual ascent. Common adverse reactions include dizziness and lightheadedness.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Glaucoma
- Dosing Information
- The recommended dosage is 1 capsule (500 mg) two times a day. Usually 1 capsule is administered in the morning and 1 capsule in the evening.
- In those unusual instances where adequate control is not obtained by the twice-a-day administration of acetazolamide, the desired control may be established by means of acetazolamide (tablets or parenteral). Use tablets or parenteral in accordance with the more frequent dosage schedules recommended for these dosage forms, such as 250 mg every four hours, or an initial dose of 500 mg followed by 250 mg or 125 mg every four hours, depending on the case in question.
Acute Mountain Sickness
- Dosing Information
- The recommended dosage is 500 mg to 1000 mg daily, in divided doses using tablets or extended-release capsules as appropriate. In circumstances of rapid ascent, such as in rescue or military operations, the higher dose level of 1000 mg is recommended. It is preferable to initiate dosing 24 to 48 hours before ascent and to continue for 48 hours while at high altitude, or longer as necessary to control symptoms.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of acetazolamide in adult patients.
Non–Guideline-Supported Use
Glaucoma Following Surgery
- Dosing Information
- 500 mg PO 1 hour postoperatively[1]
Macular Retinal Edema
- Dosing Information
- 500 mg PO qd for 4 weeks[2]
Periodic Ataxia
- Dosing Information
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
The safety and effectiveness of acetazolamide in pediatric patients below the age of 12 years have not been established. Growth retardation has been reported in children receiving long-term therapy, believed secondary to chronic acidosis.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of acetazolamide in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of acetazolamide in pediatric patients.
Contraindications
- Hypersensitivity to acetazolamide or any excipients in the formulation.
- Since acetazolamide is a sulfonamide derivative, cross sensitivity between acetazolamide, sulfonamides and other sulfonamide derivatives is possible.
- Acetazolamide therapy is contraindicated in situations in which sodium and/or potassium blood serum levels are depressed, in cases of marked kidney and liver disease or dysfunction, in suprarenal gland failure, and in hyperchloremic acidosis.
- Acetazolamide is contraindicated in patients with cirrhosis because of the risk of development of hepatic encephalopathy.
- Chronic non-congestive angle-closure glaucoma
- Long-term administration of acetazolamide is contraindicated in patients with chronic non-congestive angle-closure glaucoma since it may permit organic closure of the angle to occur while the worsening glaucoma is masked by lowered intraocular pressure.
Warnings
- Fatalities have occurred, although rarely, due to severe reactions to sulfonamides including Stevens-Johnson syndrome, toxic epidermal necrolysis, fulminant hepatic necrosis, anaphylaxis, agranulocytosis, aplastic anemia, and other blood dyscrasias.
- Sensitizations may recur when a sulfonamide is readministered irrespective of the route of administration. If signs of hypersensitivity or other serious reactions occur, discontinue use of this drug.
- Caution is advised for patients receiving concomitant high-dose aspirin and acetazolamide, as anorexia, tachypnea, lethargy, metabolic acidosis, coma, and death have been reported.
- Increasing the dose does not increase the diuresis and may increase the incidence of drowsiness and/or paresthesia. Increasing the dose often results in a decrease in diuresis. Under certain circumstances, however, very large doses have been given in conjunction with other diuretics in order to secure diuresis in complete refractory failure.
- To monitor for hematologic reactions common to all sulfonamides, it is recommended that a baseline CBC and platelet count be obtained on patients prior to initiating acetazolamide therapy and at regular intervals during therapy. If significant changes occur, early discontinuance and institution of appropriate therapy are important. Periodic monitoring of serum electrolytes is recommended.
Adverse Reactions
Clinical Trials Experience
There is limited information regarding Clinical Trial Experience of acetazolamide in the drug label.
Postmarketing Experience
Body as a whole
Headache, malaise, fatigue, fever, pain at injection site, flushing, growth retardation in children, flaccid paralysis, anaphylaxis.
Digestive
Gastrointestinal disturbances such as nausea, vomiting, diarrhea.
Hematological/Lymphatic
Blood dyscrasias such as aplastic anemia, agranulocytosis, leukopenia, thrombocytopenic purpura, melena.
Hepato-biliary disorders
Abnormal liver function, cholestatic jaundice, hepatic insufficiency, fulminant hepatic necrosis.
Metabolic/Nutritional
Metabolic acidosis, electrolyte imbalance, including hypokalemia, hyponatremia, osteomalacia with long-term phenytoin therapy, loss of appetite, taste alteration, hyper/hypoglycemia.
Nervous
Drowsiness, paresthesia (including numbness and tingling of extremities and face), depression, excitement, ataxia, confusion, convulsions, dizziness.
Skin
Allergic skin reactions including urticaria, photosensitivity, Stevens-Johnson syndrome, toxic epidermal necrolysis.
Special senses
Hearing disturbances, tinnitus, transient myopia.
Drug Interactions
- Caution is advised for patients receiving concomitant high-dose aspirin and acetazolamide, as anorexia, tachypnea, lethargy, metabolic acidosis, coma, and death have been reported.
- Acetazolamide modifies phenytoin metabolism with increased serum levels of phenytoin. This may increase or enhance the occurrence of osteomalacia in some patients receiving chronic phenytoin therapy. Caution is advised in patients receiving chronic concomitant therapy. By decreasing the gastrointestinal absorption of primidone, acetazolamide may decrease serum concentrations of primidone and its metabolites, with a consequent possible decrease in anticonvulsant effect. Caution is advised when beginning, discontinuing, or changing the dose of acetazolamide in patients receiving primidone.
- Carbonic anhydrase inhibitors
- Because of possible additive effects with other carbonic anhydrase inhibitors, concomitant use is not advisable.
- Folic acid antagonists
- Acetazolamide may increase the effects of other folic acid antagonists.
- Acetazolamide decreases urinary excretion of amphetamine and may enhance the magnitude and duration of their effect.
- Acetazolamide reduces urinary excretion of quinidine and may enhance its effect.
- Acetazolamide may prevent the urinary antiseptic effect of methenamine.
- Acetazolamide increases lithium excretion and the lithium may be decreased.
- Acetazolamide and sodium bicarbonate used concurrently increase the risk of renal calculus formation.
- Acetazolamide may elevate cyclosporine levels.
- Drug/laboratory test interactions
- Sulfonamides may give false negative or decreased values for urinary phenolsulfonphthalein and phenol red elimination values for urinary protein, serum non-protein, and serum uric acid.
- Acetazolamide may produce an increased level of crystals in the urine.
- Acetazolamide interferes with the HPLC method of assay for theophylline. Interference with the theophylline assay by acetazolamide depends on the solvent used in the extraction; acetazolamide may not interfere with other assay methods for theophylline.
Use in Specific Populations
Pregnancy
Teratogenic effects
acetazolamide, administered orally or parenterally, has been shown to be teratogenic (defects of the limbs) in mice, rats, hamsters, and rabbits. There are no adequate and well-controlled studies in pregnant women. Acetazolamide should be used in pregnancy only if the potential benefit justifies the potential risk to the fetus.
Pregnancy Category (AUS): B3
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Acetazolamide (extended-release capsule) in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Acetazolamide (extended-release capsule) during labor and delivery.
Nursing Mothers
Because of the potential for serious adverse reactions in nursing infants from acetazolamide, a decision should be made whether to discontinue nursing or to discontinue the drug taking into account the importance of the drug to the mother. Acetazolamide should only be used by nursing women if the potential benefit justifies the potential risk to the child.
Pediatric Use
The safety and effectiveness of acetazolamide in pediatric patients below the age of 12 years have not been established. Growth retardation has been reported in children receiving long-term therapy, believed secondary to chronic acidosis.
Geriatic Use
Metabolic acidosis, which can be severe, may occur in the elderly with reduced renal function. In general, dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and concomitant disease or other drug therapy.
Gender
There is no FDA guidance on the use of Acetazolamide (extended-release capsule) with respect to specific gender populations.
Race
There is no FDA guidance on the use of Acetazolamide (extended-release capsule) with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Acetazolamide (extended-release capsule) in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Acetazolamide (extended-release capsule) in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Acetazolamide (extended-release capsule) in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Acetazolamide (extended-release capsule) in patients who are immunocompromised.
Administration and Monitoring
Administration
Oral
Monitoring
Electrolyte Imbalances
Acetazolamide treatment may cause electrolyte imbalances, including hyponatremia and hypokalemia, as well as metabolic acidosis. Therefore, periodic monitoring of serum electrolytes is recommended.
Hematologic Distrubances
To monitor for hematologic reactions common to all sulfonamides, it is recommended that a baseline CBC and platelet count be obtained on patients prior to initiating acetazolamide therapy and at regular intervals during therapy. If significant changes occur, early discontinuance and institution of appropriate therapy are important.
IV Compatibility
There is limited information regarding IV Compatibility of acetazolamide in the drug label.
Overdosage
Acute Overdose
Management
- No specific antidote is known.
- Treatment should be symptomatic and supportive. Electrolyte imbalance, development of an acidotic state, and central nervous system effects might be expected to occur. Serum electrolyte levels (particularly potassium) and blood pH levels should be monitored. Supportive measures are required to restore electrolyte and pH balance. The acidotic state can usually be corrected by the administration of bicarbonate.
- Despite its high intraerythrocytic distribution and plasma protein binding properties, acetazolamide may be dialyzable. This may be particularly important in the management of acetazolamide overdosage when complicated by the presence of renal failure.
Pharmacology
| |
| |
Acetazolamide (extended-release capsule)
| |
| Systematic (IUPAC) name | |
| N-(5-sulfamoyl-1,3,4-thiadiazol-2-yl)acetamide | |
| Identifiers | |
| CAS number | |
| ATC code | S01 |
| PubChem | |
| DrugBank | |
| Chemical data | |
| Formula | Template:OrganicBox atomTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox |
| Mol. mass | 222.245 g/mol |
| SMILES | & |
| Pharmacokinetic data | |
| Bioavailability | ? |
| Metabolism | None |
| Half life | 2-4 hours |
| Excretion | Urine (90%) |
| Therapeutic considerations | |
| Pregnancy cat. | |
| Legal status |
Prescription Only (S4)(AU) ?(CA) POM(UK) [[Prescription drug|Template:Unicode-only]](US) |
| Routes | oral or intravenous |
Mechanism of Action
- Acetazolamide is a potent carbonic anhydrase inhibitor, effective in the control of fluid secretion (e.g., some types of glaucoma), in the treatment of certain convulsive disorders (e.g., epilepsy), and in the promotion of diuresis in instances of abnormal fluid retention (e.g., cardiac edema).
- Acetazolamide is not a mercurial diuretic. Rather, it is a non-bacteriostatic sulfonamide possessing a chemical structure and pharmacological activity distinctly different from the bacteriostatic sulfonamides.
- Acetazolamide is an enzyme inhibitor that acts specifically on carbonic anhydrase, the enzyme that catalyzes the reversible reaction involving the hydration of carbon dioxide and the dehydration of carbonic acid. In the eye, this inhibitory action of acetazolamide decreases the secretion of aqueous humor and results in a drop in intraocular pressure, a reaction considered desirable in cases of glaucoma and even in certain non-glaucomatous conditions. Evidence seems to indicate that acetazolamide has utility as an adjuvant in treatment of certain dysfunctions of the central nervous system (e.g., epilepsy). Inhibition of carbonic anhydrase in this area appears to retard abnormal, paroxysmal, excessive discharge from central nervous system neurons. The diuretic effect of acetazolamide is due to its action in the kidney on the reversible reaction involving hydration of carbon dioxide and dehydration of carbonic acid. The result is renal loss of HCO3 ion, which carries out sodium, water, and potassium. Alkalinization of the urine and promotion of diuresis are thus affected. Alteration in ammonia metabolism occurs due to increased reabsorption of ammonia by the renal tubules as a result of urinary alkalinization.
Structure
The chemical name for acetazolamide is N-(5-Sulfamoyl-1,3, 4-thiadiazol-2-yl) acetamide and has the following chemical structure:

Pharmacodynamics
- Acetazolamide provide prolonged action to inhibit aqueous humor secretion for 18 to 24 hours after each dose, whereas tablets act for only eight to 12 hours. The prolonged continuous effect of acetazolamide permits a reduction in dosage frequency.
Pharmacokinetics
- Plasma concentrations of acetazolamide peak from three to six hours after administration of acetazolamide, compared to one to four hours with tablets. Food does not affect bioavailability of acetazolamide.
- Placebo-controlled clinical trials have shown that prophylactic administration of acetazolamide at a dose of 250 mg every eight to 12 hours (or a 500 mg controlled release capsule once daily) before and during rapid ascent to altitude results in fewer and/or less severe symptoms of acute mountain sickness (AMS) such as headache, nausea, shortness of breath, dizziness, drowsiness, and fatigue. Pulmonary function (e.g., minute ventilation, expired vital capacity, and peak flow) is greater in the acetazolamide treated group, both in subjects with AMS and asymptomatic subjects. The acetazolamide treated climbers also had less difficulty in sleeping.
Nonclinical Toxicology
There is limited information regarding Nonclinical Toxicology of acetazolamide in the drug label.
Clinical Studies
There is limited information regarding Clinical Studies of acetazolamide in the drug label.
How Supplied
- Diamox Sequels (Acetazolamide Extended-Release Capsules) are available as 500 mg:
- Orange opaque cap and orange opaque body filled with white to off-white pellets. Imprinted in black ink, DIAMOX 754.
- Available in bottles of:
- 100 NDC 51285-754-02
- DURAMED PHARMACEUTICALS, INC.
- Subsidiary of Barr Pharmaceuticals, Inc.
- Pomona, New York 10970
- Revised JULY 2008
- BR-754
Storage
Store at 20° to 25°C (68° to 77°F).
Images
Drug Images
{{#ask: Page Name::Acetazolamide (extended-release capsule) |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel


{{#ask: Label Page::Acetazolamide (extended-release capsule) |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
- Adverse reactions common to all sulfonamide derivatives may occur: anaphylaxis, fever, rash (including erythema multiforme, Stevens-Johnson syndrome, toxic epidermal necrolysis), crystalluria, renal calculus, bone marrow depression, thrombocytopenic purpura, hemolytic anemia, leukopenia, pancytopenia, and agranulocytosis. Caution is advised for early detection of such reactions and the drug should be discontinued and appropriate therapy instituted.
- In patients with pulmonary obstruction or emphysema where alveolar ventilation may be impaired, acetazolamide which may precipitate or aggravate acidosis should be used with caution.
- Gradual ascent is desirable to try to avoid acute mountain sickness. If rapid ascent is undertaken and acetazolamide is used, it should be noted that such use does not obviate the need for prompt descent if severe forms of high altitude sickness occur, i.e., high altitude pulmonary edema (HAPE) or high altitude cerebral edema.
- Caution is advised for patients receiving concomitant high-dose aspirin and acetazolamide, as anorexia, tachypnea, lethargy, metabolic acidosis, coma, and death have been reported.
- Both increases and decreases in blood glucose have been described in patients treated with acetazolamide. This should be taken into consideration in patients with impaired glucose tolerance or diabetes mellitus.
- Acetazolamide treatment may cause electrolyte imbalances, including hyponatremia and hypokalemia, as well as metabolic acidosis. Therefore, periodic monitoring of serum electrolytes is recommended. Particular caution is recommended in patients with conditions that are associated with, or predispose a patient to, electrolyte and acid/base imbalances, such as patients with impaired renal function (including elderly patients), patients with diabetes mellitus, and patients with impaired alveolar ventilation.
- Some adverse reactions to acetazolamide, such as drowsiness, fatigue, and myopia, may impair the ability to drive and operate machinery.[7]
Precautions with Alcohol
Alcohol-acetazolamide interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
Diamox Sequels®
Look-Alike Drug Names
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
- ↑ Byrd, S.; Singh, K. (1998). "Medical control of intraocular pressure after cataract surgery". J Cataract Refract Surg. 24 (11): 1493–7. PMID 9818340. Unknown parameter
|month=ignored (help) - ↑ Whitcup, SM.; Csaky, KG.; Podgor, MJ.; Chew, EY.; Perry, CH.; Nussenblatt, RB. (1996). "A randomized, masked, cross-over trial of acetazolamide for cystoid macular edema in patients with uveitis". Ophthalmology. 103 (7): 1054–62, discussion 1062-3. PMID 8684794. Unknown parameter
|month=ignored (help) - ↑ Baloh, RW.; Winder, A. (1991). "acetazolamide-responsive vestibulocerebellar syndrome: clinical and oculographic features". Neurology. 41 (3): 429–33. PMID 2006014. Unknown parameter
|month=ignored (help) - ↑ Friedman, JH.; Hollmann, PA. (1987). "acetazolamide responsive hereditary paroxysmal ataxia". Mov Disord. 2 (1): 67–72. doi:10.1002/mds.870020110. PMID 3332806.
- ↑ Bouchard, JP.; Roberge, C.; van Gelder, NM.; Barbeau, A. (1984). "Familial periodic ataxia responsive to acetazolamide". Can J Neurol Sci. 11 (4 Suppl): 550–3. PMID 6439406. Unknown parameter
|month=ignored (help) - ↑ Griggs, RC.; Moxley, RT.; Lafrance, RA.; McQuillen, J. (1978). "Hereditary paroxysmal ataxia: response to acetazolamide". Neurology. 28 (12): 1259–64. PMID 366453. Unknown parameter
|month=ignored (help) - ↑ "DIAMOX SEQUELS (ACETAZOLAMIDE) CAPSULE, EXTENDED RELEASE [DURAMED PHARMACEUTICALS, INC.]".
- ↑ 8.0 8.1 "http://www.ismp.org". External link in
|title=(help)
{{#subobject:
|Page Name=Acetazolamide (extended-release capsule) |Pill Name=Diamox_Sequels_NDC_512850754.jpg |Drug Name=Diamox Sequels |Pill Ingred=ACETAZOLAMIDE[ACETAZOLAMIDE]|+sep=; |Pill Imprint=DIAMOX;754 |Pill Dosage=500 mg |Pill Color=Orange|+sep=; |Pill Shape=Capsule |Pill Size (mm)=23 |Pill Scoring=1 |Pill Image= |Drug Author=Duramed Pharmaceuticals, Inc. |NDC=512850754
}}
{{#subobject:
|Page Name=Acetazolamide (extended-release capsule) |Pill Name=Acetazolamide_NDC_516724022.jpg |Drug Name=Acetazolamide |Pill Ingred=Acetazolamide[Acetazolamide]|+sep=; |Pill Imprint=T52 |Pill Dosage=125 mg |Pill Color=White|+sep=; |Pill Shape=Round |Pill Size (mm)=9 |Pill Scoring=2 |Pill Image= |Drug Author=Taro Pharmaceuticals U.S.A., Inc. |NDC=516724022
}}
{{#subobject:
|Page Name=Acetazolamide (extended-release capsule) |Pill Name=Acetazolamide_NDC_516724023.jpg |Drug Name=Acetazolamide |Pill Ingred=Acetazolamide[Acetazolamide]|+sep=; |Pill Imprint=T53 |Pill Dosage=250 mg |Pill Color=White|+sep=; |Pill Shape=Round |Pill Size (mm)=11 |Pill Scoring=4 |Pill Image= |Drug Author=Taro Pharmaceuticals U.S.A., Inc. |NDC=516724023
}}
{{#subobject:
|Page Name=Acetazolamide (extended-release capsule) |Pill Name=AcetaZOLAMIDE_NDC_604290033.jpg |Drug Name=AcetaZOLAMIDE |Pill Ingred=Acetazolamide[Acetazolamide]|+sep=; |Pill Imprint=T52 |Pill Dosage=125 mg |Pill Color=White|+sep=; |Pill Shape=Round |Pill Size (mm)=9 |Pill Scoring=2 |Pill Image= |Drug Author=Golden State Medical Supply, Inc. |NDC=604290033
}}
{{#subobject:
|Page Name=Acetazolamide (extended-release capsule) |Pill Name=AcetaZOLAMIDE_NDC_604290034.jpg |Drug Name=AcetaZOLAMIDE |Pill Ingred=Acetazolamide[Acetazolamide]|+sep=; |Pill Imprint=T53 |Pill Dosage=250 mg |Pill Color=White|+sep=; |Pill Shape=Round |Pill Size (mm)=11 |Pill Scoring=4 |Pill Image= |Drug Author=Golden State Medical Supply, Inc. |NDC=604290034
}}
{{#subobject:
|Page Name=Acetazolamide (extended-release capsule) |Pill Name=AcetaZOLAMIDE_NDC_05271050.jpg |Drug Name=AcetaZOLAMIDE |Pill Ingred=ACETAZOLAMIDE[ACETAZOLAMIDE]|+sep=; |Pill Imprint=LAN;1050 |Pill Dosage=250 mg |Pill Color=White|+sep=; |Pill Shape=Round |Pill Size (mm)=11 |Pill Scoring=4 |Pill Image= |Drug Author=Lannett Company, Inc. |NDC=05271050
}}
{{#subobject:
|Page Name=Acetazolamide (extended-release capsule) |Pill Name=Acetazolamide_NDC_05550513.jpg |Drug Name=Acetazolamide |Pill Ingred=ACETAZOLAMIDE[ACETAZOLAMIDE]|+sep=; |Pill Imprint=barr;513 |Pill Dosage=500 mg |Pill Color=Orange|+sep=; |Pill Shape=Capsule |Pill Size (mm)=23 |Pill Scoring=1 |Pill Image= |Drug Author=Barr Laboratories Inc.. |NDC=05550513
}}
{{#subobject:
|Page Name=Acetazolamide (extended-release capsule) |Pill Name=Acetazolamide_NDC_683820261.jpg |Drug Name=Acetazolamide |Pill Ingred=ACETAZOLAMIDE[ACETAZOLAMIDE]|+sep=; |Pill Imprint=EP;107 |Pill Dosage=500 mg |Pill Color=|+sep=; |Pill Shape=Capsule |Pill Size (mm)=25 |Pill Scoring=1 |Pill Image= |Drug Author=Zydus Pharmaceuticals (USA) Inc. |NDC=683820261
}}
{{#subobject:
|Label Page=Acetazolamide (extended-release capsule) |Label Name=DIAMOX SEQUELS (acetazolamide) capsule, extended release label display.jpeg
}}
{{#subobject:
|Label Page=Acetazolamide (extended-release capsule) |Label Name=Acetazolamide004.jpg
}}