Loefflers syndrome pathophysiology
Jump to navigation
Jump to search
|
Löffler's syndrome Microchapters |
|
Diagnosis |
|---|
|
Treatment |
|
Case Studies |
|
Loefflers syndrome pathophysiology On the Web |
|
American Roentgen Ray Society Images of Loefflers syndrome pathophysiology |
|
Risk calculators and risk factors for Loefflers syndrome pathophysiology |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]Associate Editor(s)-in-Chief: Soroush Seifirad, M.D.[2]
Overview
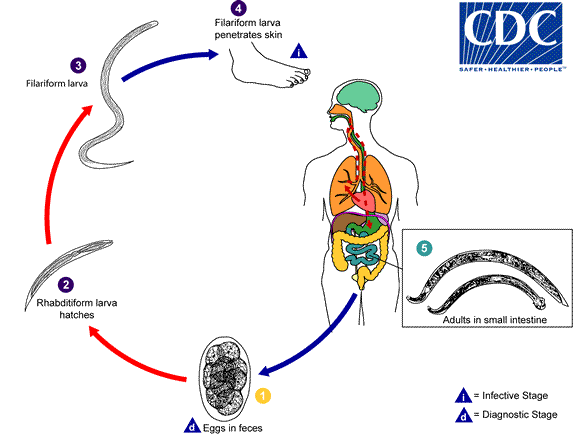
It is understood that Löffler syndrome is the result of transpulmonary passage of helminth larvae. Helminths, with a pulmonary life cycle are responsible for this syndrome, among them are Ascaris lumbricoides, Ascaris suum, Ancylostoma duodenale, Necator americanus, and Strongyloides stercoralis.
Pathophysiology
Pathogenesis
- It is understood that Löffler syndrome is the result of the transpulmonary passage of helminth larvae. Helminths, with a pulmonary life cycle are responsible for this acute hypersensitivity reaction syndrome, among them are Ascaris lumbricoides, Ascaris suum, Ancylostoma duodenale, Necator americanus, and Strongyloides stercoralis.
- Pathogen is usually transmitted via the oral route (Ascaris) or penetrate the skin (Necator) to the human host.[1][2][3][4][5]
- Following transmission/ingestion, infecting larvae reach the lungs via the bloodstream, penetrate into alveoli, mature, and ascend the airways before descending the alimentary tract into the small bowel
- Ascaris is the most common cause of Löffler syndrome worldwide. On the other hand, migrating larvae of hookworms (Ancylostoma duodenale, Necator americanus) and Strongyloides are less likely to elicit symptoms or pulmonary eosinophilia.

- Pulmonary eosinophilia is a different term, with a wider range of infectious and noninfectious pathophysiology.
- Parasites without pulmonary cycle has been linked to pulmonary eosinophilia.
- It is believed that pulmonary eosinophilia is a hypersensitivity reaction due to circulating, but not local inflammatory mediators such as likeinterleukin-5 (IL-5).
- Since challenged athymic mice have not developed pulmonary eosinophilia, it has been suggested that pulmonary eosinophilia is aT cell-dependent phenomenon.
Associated Conditions
Conditions associated with Loeffler syndrome include subjects infected with:
- Ascaris lumbricoides,
- Ascaris suum, Ancylostoma duodenale,
- Necator americanus,
- Strongyloides stercoralis.
Gross Pathology
- Loeffler syndrome has its own characteristic imaging findings.
- Biopsy is rarely performed
- Diagnosis is based on clinical findings, radiographic findings, and shreds of evidence of parasite presence in pulmonary secretions.
- Blood-tinged sputum might be presented.
Microscopic Pathology
- Eosinophils may be present in sputum
- Eosinophil-derived Charcot-Leyden crystals may be present in blood-tinged sputum
- Stool examinations are generally negative at the time of pulmonary symptoms and thus not useful in the diagnosis of Löffler syndrome.
- Ascaris, Strongyloides, or hookworm larvae might be detected in the respiratory secretions


- Since biopsy is not indicated in patients with the lofeffler syndrome, reported pathological presentations of Loeffler syndrome are based on the autopsy of patients who passed away from another cause while they concomitantly had simple pulmonary eosinophilia.
- It has been shown that eosinophilic infiltration occurs in the bronchi and bronchioles and in the alveolar and interstitial spaces.
- Usually no parasitic form has been found in the lungs.[6][7]
References
- ↑ Dold C, Holland CV (2011) Ascaris and ascariasis. Microbes Infect 13 (7):632-7. DOI:10.1016/j.micinf.2010.09.012 PMID: 20934531
- ↑ Hoagland KE, Schad GA (1978) Necator americanus and Ancylostoma duodenale: life history parameters and epidemiological implications of two sympatric hookworms of humans. Exp Parasitol 44 (1):36-49. PMID: 627275
- ↑ Brooker S, Bethony J, Hotez PJ (2004) Human hookworm infection in the 21st century. Adv Parasitol 58 ():197-288. DOI:10.1016/S0065-308X(04)58004-1 PMID: 15603764
- ↑ Page W, Judd JA, Bradbury RS (2018) The Unique Life Cycle of Strongyloides stercoralis and Implications for Public Health Action. Trop Med Infect Dis 3 (2):. DOI:10.3390/tropicalmed3020053 PMID: 30274449
- ↑ Nutman TB (2017) Human infection with Strongyloides stercoralis and other related Strongyloides species. Parasitology 144 (3):263-273. DOI:10.1017/S0031182016000834 PMID: 27181117
- ↑ Caulet T (1957) [Loffler syndrome and pulmonary eosinophilia.] Gaz Med Fr 64 (20):1737-8 passim. PMID: 13480465
- ↑ (1968) Löffler's syndrome. Br Med J 3 (5618):569-70. PMID: 5667987