Gemifloxacin mesylate
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Kiran Singh, M.D. [2]
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Black Box Warning
|
WARNING:
See full prescribing information for complete Boxed Warning.
* Fluoroquinolones, including FACTIVE®, are associated with an increased risk of tendinitis and tendon rupture in all ages. This risk is further increased in older patients usually over 60 years of age, in patients taking corticosteroid drugs, and in patients with kidney, heart and lung transplants.
|
Overview
Gemifloxacin mesylate is an antibiotic that is FDA approved for the treatment of acute bacterial exacerbation of chronic bronchitis,community-acquired pneumonia. There is a Black Box Warning for this drug as shown here. Common adverse reactions include rash, abdominal pain, diarrhea, nausea, vomiting,dizziness, headache.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Indications
Gemifloxacin mesylate is indicated for the treatment of infections caused by susceptible strains of the designated microorganisms in the conditions listed below.
Acute bacterial exacerbation of chronic bronchitis caused by Streptococcus pneumoniae, Haemophilus influenzae, Haemophilus parainfluenzae, or Moraxella catarrhalis.
Community-acquired pneumonia (of mild to moderate severity) caused by Streptococcus pneumoniae (including multi-drug resistant strains [MDRSP])*, Haemophilus influenzae, Moraxella catarrhalis, Mycoplasma pneumoniae, Chlamydia pneumoniae, or Klebsiella pneumoniae.
MDRSP: multi-drug resistant Streptococcus pneumoniae, includes isolates previously known as PRSP (penicillin-resistant Streptococcus pneumoniae), and are strains resistant to two or more of the following antibiotics: penicillin (MIC ≥2 μg/mL), 2nd generation cephalosporins (e.g., cefuroxime), macrolides, tetracyclines and trimethoprim/sulfamethoxazole.
To reduce the development of drug-resistant bacteria and maintain the effectiveness of gemifloxacin mesylate and other antibacterial drugs,gemifloxacin mesylate should be used only to treat infections that are proven or strongly suspected to be caused by susceptible bacteria. When culture and susceptibility information are available, they should be considered in selecting or modifying antibacterial therapy. In the absence of such data, local epidemiology and susceptibility patterns may contribute to the empiric selection of therapy.
Dosage
Gemifloxacin mesylate can be taken with or without food and should be swallowed whole with a liberal amount of liquid. The recommended dose of gemifloxacin mesylate is 320 mg daily, according to the following table (Table 4).
Recommended Dosage Regimen of Gemifloxacin Mesylate
- The clinical decision regarding the use of a 5 or 7 day regimen should be guided by results of the initial sputum culture.

The recommended dose and duration of gemifloxacin mesylate should not be exceeded.
Use in Renally Impaired Patients: Dose adjustment in patients with creatinine clearance >40 mL/min is not required. Modification of the dosage is recommended for patients with creatinine clearance ≤40 mL/min. Table 5 provides dosage guidelines for use in patients with renal impairment.

- Patients requiring routine hemodialysis or continuous ambulatory peritoneal dialysis (CAPD) should receive 160 mg every 24 hours.
- When only the serum creatinine concentration is known, the following formula may be used to estimate creatinine clearance.

Creatinine Clearance Formula
Women: 0.85 x the value calculated for men
Use in Hepatically Impaired Patients: No dosage adjustment is recommended in patients with mild (Child-Pugh Class A), moderate (Child-Pugh Class B) or severe (Child-Pugh Class C) hepatic impairment.
Use in Elderly: No dosage adjustment is recommended.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Gemifloxacin mesylate in adult patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Gemifloxacin mesylate in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
There is limited information regarding FDA-Labeled Use of Gemifloxacin mesylate in pediatric patients.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Gemifloxacin mesylate in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Gemifloxacin mesylate in pediatric patients.
Contraindications
- Gemifloxacin mesylate is contraindicated in patients with a history of hypersensitivity to gemifloxacin, fluoroquinolone antibiotic agents, or any of the product components.
Warnings
|
WARNING:
See full prescribing information for complete Boxed Warning.
* Fluoroquinolones, including FACTIVE®, are associated with an increased risk of tendinitis and tendon rupture in all ages. This risk is further increased in older patients usually over 60 years of age, in patients taking corticosteroid drugs, and in patients with kidney, heart and lung transplants.
|
Tendinopathy and Tendon Rupture:
- Fluoroquinolones, including gemifloxacin mesylate, are associated with an increased risk of tendinitis and tendon rupture in all ages. This adverse reaction most frequently involves the Achilles tendon, and rupture of the Achilles tendon may require surgical repair. Tendinitis and tendon rupture in the rotator cuff (the shoulder), the hand, the biceps, the thumb, and other tendon sites have also been reported. The risk of developing fluoroquinolone-associated tendinitis and tendon rupture is further increased in older patients usually over 60 years of age, in those taking corticosteroid drugs, and in patients with kidney, heart or lung transplants. Factors, in addition to age and corticosteroid use, that may independently increase the risk of tendon rupture include strenuous physical activity, renal failure, and previous tendon disorders such as rheumatoid arthritis. Tendinitis and tendon rupture have also occurred in patients taking fluoroquinolones who do not have the above risk factors. Tendon rupture can occur during or after completion of therapy; cases occurring up to several months after completion of therapy have been reported. Gemifloxacin mesylate should be discontinued if the patient experiences pain, swelling, inflammation or rupture of a tendon. Patients should be advised to rest at the first sign of tendinitis or tendon rupture, and to contact their healthcare provider regarding changing to a non-quinolone antimicrobial drug.
Exacerbation of Myasthenia Gravis:
- Fluoroquinolones, including gemifloxacin mesylate, have neuromuscular blocking activity and may exacerbate muscle weakness in persons with myasthenia gravis. Postmarketing serious adverse events, including deaths and requirement for ventilatory support, have been associated with fluoroquinolone use in persons with myasthenia gravis. Avoid gemifloxacin mesylate in patients with known history of myasthenia gravis.
THE SAFETY AND EFFECTIVENESS OF GEMIFLOXACIN MESYLATE IN CHILDREN, ADOLESCENTS (LESS THAN 18 YEARS OF AGE), PREGNANT WOMEN, AND LACTATING WOMEN HAVE NOT BEEN ESTABLISHED.
QT Effects:
- Fluoroquinolones may prolong the QT interval in some patients. Gemifloxacin mesylate should be avoided in patients with a history of prolongation of the QTc interval, patients with uncorrected electrolyte disorders (hypokalemia or hypomagnesemia), and patients receiving Class IA (e.g., quinidine, procainamide) or Class III (e.g., amiodarone, sotalol) antiarrhythmic agents.
- Pharmacokinetic studies between gemifloxacin and drugs that prolong the QTc interval such as erythromycin, antipsychotics, and tricyclic antidepressants have not been performed. Gemifloxacin mesylate should be used with caution when given concurrently with these drugs, as well as in patients with ongoing proarrhythmic conditions, such as clinically significant bradycardia or acute myocardial ischemia. No cardiovascular morbidity or mortality attributable to QTc prolongation occurred with gemifloxacin mesylate treatment in over 8119 patients, including 707 patients concurrently receiving drugs known to prolong the QTc interval and 7 patients with hypokalemia.
- The likelihood of QTc prolongation may increase with increasing dose of the drug; therefore, the recommended dose should not be exceeded especially in patients with renal or hepatic impairment where the Cmax and AUC are slightly higher. QTc prolongation may lead to an increased risk for ventricular arrhythmias including torsades de pointes. The maximal change in the QTc interval occurs approximately 5-10 hours following oral administration of gemifloxacin.
Hypersensitivity Reactions:
- Serious hypersensitivity and/or anaphylactic reactions have been reported in patients receiving fluoroquinolone therapy, including gemifloxacin mesylate. Hypersensitivity reactions reported in patients receiving fluoroquinolone therapy have occasionally been fatal. These reactions may occur following the first dose. Some reactions have been accompanied by cardiovascular collapse, hypotension/shock, seizure, loss of consciousness, tingling, angioedema (including tongue, laryngeal, throat or facial edema/swelling), airway obstruction (including bronchospasm, shortness of breath and acute respiratory distress), dyspnea, urticaria, itching and other serious skin reactions.
- Gemifloxacin mesylate should be discontinued immediately at the appearance of any sign of an immediate type I hypersensitivity skin rash or any other manifestation of a hypersensitivity reaction; the need for continued fluoroquinolone therapy should be evaluated. As with other drugs, serious acute hypersensitivity reactions may require treatment with epinephrine and other resuscitative measures, including oxygen, intravenous fluids, antihistamines, corticosteroids, pressor amines and airway management as clinically indicated.
- Other serious and sometimes fatal events, some due to hypersensitivity and some due to uncertain etiology, have been reported rarely in patients receiving therapy with quinolones, including gemifloxacin mesylate. These events may be severe and generally occur following the administration of multiple doses. Clinical manifestations may include one or more of the following:
- Fever, rash or severe dermatologic reactions (e.g., toxic epidermal necrolysis, Stevens-Johnson Syndrome);
- Vasculitis; arthralgia; myalgia; serum sickness;
- Allergic pneumonitis;
- Interstitial nephritis; acute renal insufficiency or failure;
- Hepatitis; jaundice; acute hepatic necrosis or failure;
- Anemia, including hemolytic and aplastic;
- Thrombocytopenia, including thrombotic thrombocytopenic purpura; leukopenia agranulocytosis; pancytopenia; and/or other hematologic abnormalities.
- The drug should be discontinued immediately at the first appearance of a skin rash, jaundice, or any other sign of hypersensitivity and supportive measures instituted .
Peripheral Neuropathy:
- Cases of sensory or sensorimotor axonal polyneuropathy affecting small and/or large axons resulting in paresthesias, hypoesthesias, dysesthesias and weakness have been reported in patients receiving fluoroquinolones, including gemifloxacin mesylate. Symptoms may occur soon after initiation of gemifloxacin mesylate and may be irreversible. gemifloxacin mesylate should be discontinued immediately if the patient experiences symptoms of peripheral neuropathy, including pain, burning, tingling, numbness, and/or weakness or other alterations in sensations including light touch, pain, temperature, position sense, and vibratory sensation.
CNS Effects:
- In clinical studies with gemifloxacin mesylate, central nervous system (CNS) effects have been reported infrequently. As with other fluoroquinolones, gemifloxacin mesylate should be used with caution in patients with CNS diseases such as epilepsy or patients predisposed to convulsions. Although not seen in gemifloxacin mesylate clinical trials, convulsions, increased intracranial pressure (including pseudotumor cerebri), and toxic psychosis have been reported in patients receiving other fluoroquinolones. CNS stimulation which may lead to tremors, restlessness, anxiety, lightheadedness, confusion, hallucinations, paranoia, depression, insomnia, and rarely suicidal thoughts or acts may also be caused by other fluoroquinolones. If these reactions occur in patients receiving gemifloxacin mesylate, the drug should be discontinued and appropriate measures instituted.
Clostridium difficile Associated Diarrhea:
- Clostridium difficile associated diarrhea (CDAD) has been reported with use of nearly all antibacterial agents, including gemifloxacin mesylate, and may range in severity from mild diarrhea to fatal colitis. Treatment with antibacterial agents alters the normal flora of the colon leading to overgrowth of C. difficile.
- C. difficile produces toxins A and B which contribute to the development of CDAD. Hypertoxin producing strains of C. difficile cause increased morbidity and mortality, as these infections can be refractory to antimicrobial therapy and may require colectomy. CDAD must be considered in all patients who present with diarrhea following antibiotic use. Careful medical history is necessary since CDAD has been reported to occur over two months after the administration of antibacterial agents.
- If CDAD is suspected or confirmed, ongoing antibiotic use not directed against C. difficile may need to be discontinued. Appropriate fluid and electrolyte management, protein supplementation, antibiotic treatment of C. difficile, and surgical evaluation should be instituted as clinically indicated.
Adverse Reactions
Clinical Trials Experience
- In clinical studies, 8119 patients received daily oral doses of 320 mg gemifloxacin mesylate. In addition, 1797 healthy volunteers and 81 patients with renal or hepatic impairment received single or repeat doses of gemifloxacin in clinical pharmacology studies. The majority of adverse reactions experienced by patients in clinical trials were considered to be of mild to moderate severity.
- Gemifloxacin mesylate was discontinued because of an adverse event (determined by the investigator to be possibly or probably related to drug) in 2.0% of patients, primarily due to rash (0.8%), nausea (0.3%), diarrhea (0.3%), urticaria (0.2%) and vomiting (0.2%). Comparator antibiotics were discontinued because of an adverse event at an overall comparable rate of 2.1%, primarily due to diarrhea (0.5%), nausea (0.4%), vomiting (0.3%), rash (0.3%), abdominal pain (0.2%) and vertigo (0.2%).
- The most commonly reported adverse events with a frequency of ≥2% for patients receiving 320 mg gemifloxacin mesylate versus comparator drug (beta-lactam antibiotics, macrolides or other fluoroquinolones) are as follows: diarrhea 5.0% vs. 6.2%; rash 3.5% vs. 1.1%; nausea 3.7% vs. 4.5%; headache 4.2% vs. 5.2%; abdominal pain 2.2% vs. 2.2%; vomiting 1.6% vs. 2.0%; and dizziness 1.7% vs. 2.6%.
Adverse Events with a Frequency of Less than 1%
- Additional drug-related adverse events (possibly or probably related) in the 8119 patients, with a frequency of >0.1% to ≤1% included: abdominal pain, anorexia, constipation, dermatitis, dizziness, dry mouth, dyspepsia, fatigue, flatulence, fungal infection, gastritis, genital moniliasis, genital pruritus, hyperglycemia, increased alkaline phosphatase, increased ALT, increased AST, increased creatine phosphokinase, insomnia, leukopenia, pruritus, somnolence, taste perversion, thrombocythemia, urticaria, vaginitis, and vomiting.
- Other adverse events reported from clinical trials which have potential clinical significance and which were considered to have a suspected relationship to the drug, that occurred in ≤0.1% of patients were: abnormal urine, abnormal vision, anemia, arthralgia, asthenia, back pain, bilirubinemia, dyspnea, eczema, eosinophilia, facial edema, flushing, gastroenteritis, granulocytopenia, hot flashes, increased GGT, increased non-protein nitrogen, leg cramps, moniliasis, myalgia, nervousness, non-specified gastrointestinal disorder, pain, pharyngitis, photosensitivity/phototoxicity.
- In clinical trials of acute bacterial exacerbation of chronic bronchitis (ABECB) and community acquired pneumonia (CAP), the incidences of rash were as follows (Table3):

Laboratory Changes:
- The percentages of patients who received multiple doses of gemifloxacin mesylate and had a laboratory abnormality are listed below. It is not known whether these abnormalities were related to gemifloxacin mesylate or an underlying condition.
- Clinical Chemistry: increased ALT (1.7%), increased AST (1.3%), increased creatine phosphokinase (0.7%), increased alkaline phosphatase (0.4%), increased total bilirubin (0.4%), increased potassium (0.3%), decreased sodium (0.2%), increased blood urea nitrogen (0.3%), decreased albumin (0.3%), increased serum creatinine (0.2%), decreased calcium (0.1%), decreased total protein (0.1%), decreased potassium (0.1%), increased sodium (0.1%), increased lactate dehydrogenase (<0.1%) and increased calcium (<0.1%).
- CPK elevations were noted infrequently: 0.7% in gemifloxacin mesylate patients vs. 0.7% in the comparator patients.
- Hematology: increased platelets (1.0%), decreased neutrophils (0.5%), increased neutrophils (0.5%), decreased hematocrit (0.3%), decreased hemoglobin (0.2%), decreased platelets (0.2%), decreased red blood cells (0.1%), increased hematocrit (0.1%), increased hemoglobin (0.1%), and increased red blood cells (0.1%).
- In clinical studies, approximately 7% of the gemifloxacin mesylate treated patients had elevated ALT values immediately prior to entry into the study. Of these patients, approximately 15% showed a further elevation of their ALT at the on-therapy visit and 9% showed a further elevation at the end of therapy visit. None of these patients demonstrated evidence of hepatocellular jaundice. For the pooled comparators, approximately 6% of patients had elevated ALT values immediately prior to entry into the study. Of these patients, approximately 7% showed a further elevation of their ALT at the on-therapy visit and 4% showed a further elevation at the end of therapy visit.
- In a clinical trial where 638 patients received either a single 640 mg dose of gemifloxacin or 250 mg BID of ciprofloxacin for 3 days, there was an increased incidence of ALT elevations in the gemifloxacin arm (3.9%) vs. the comparator arm (1.0%). In this study, two patients experienced ALT elevations of 8 to 10 times the upper limit of normal. These elevations were asymptomatic and reversible.
Postmarketing Experience
- The majority of the post-marketing adverse events reported were cutaneous and most of these were rash. Some of these cutaneous adverse events were considered serious. The majority of the rashes occurred in women and in patients under 40 years of age.
- The following are additional adverse reactions reported during the post-marketing use of gemifloxacin mesylate. Since these reactions are reported voluntarily from a population of uncertain size, it is impossible to reliably estimate their frequency or establish a causal relationship to gemifloxacin mesylate exposure:
- Peripheral neuropathy that may be irreversible;
- Uveitis;
- Anaphylactic reaction, erythema multiforme, skin exfoliation, facial swelling;
- Exacerbation of myasthenia gravis;
- Hemorrhage, increased international normalized ratio (INR), retinal hemorrhage;
- Peripheral edema;
- Renal failure;
- Prolonged QT, supraventricular tachycardia, syncope, transient ischemic attack;
- Photosensitivity/phototoxicity reaction;
- Antibiotic-associated colitis;
- Tendon rupture.
Drug Interactions
There is limited information regarding Gemifloxacin mesylate Drug Interactions in the drug label.
Use in Specific Populations
Pregnancy
- Pregnancy Category
- Australian Drug Evaluation Committee (ADEC) Pregnancy Category
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Gemifloxacin mesylate in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Gemifloxacin mesylate during labor and delivery.
Nursing Mothers
There is no FDA guidance on the use of Gemifloxacin mesylate with respect to nursing mothers.
Pediatric Use
There is no FDA guidance on the use of Gemifloxacin mesylate with respect to pediatric patients.
Geriatic Use
There is no FDA guidance on the use of Gemifloxacin mesylate with respect to geriatric patients.
Gender
There is no FDA guidance on the use of Gemifloxacin mesylate with respect to specific gender populations.
Race
There is no FDA guidance on the use of Gemifloxacin mesylate with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Gemifloxacin mesylate in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Gemifloxacin mesylate in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Gemifloxacin mesylate in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Gemifloxacin mesylate in patients who are immunocompromised.
Administration and Monitoring
Administration
Monitoring
There is limited information regarding Monitoring of Gemifloxacin mesylate in the drug label.
IV Compatibility
There is limited information regarding IV Compatibility of Gemifloxacin mesylate in the drug label.
Overdosage
- Any signs or symptoms of overdosage should be treated symptomatically. No specific antidote is known. In the event of acute oral overdosage, the stomach should be emptied by inducing vomiting or by gastric lavage; the patient should be carefully observed and treated symptomatically with appropriate hydration maintained. Hemodialysis removes approximately 20 to 30% of an oral dose of gemifloxacin from plasma.
- Mortality occurred at oral gemifloxacin doses of 1600 mg/kg in rats and 320 mg/kg in mice. The minimum lethal intravenous doses in these species were 160 and 80 mg/kg, respectively. Toxic signs after administration of a single high oral dose (400 mg/kg) of gemifloxacin to rodents included ataxia, lethargy, piloerection, tremor, and clonic convulsions.
Pharmacology
Mechanism of Action
There is limited information regarding Gemifloxacin mesylate Mechanism of Action in the drug label.
Structure
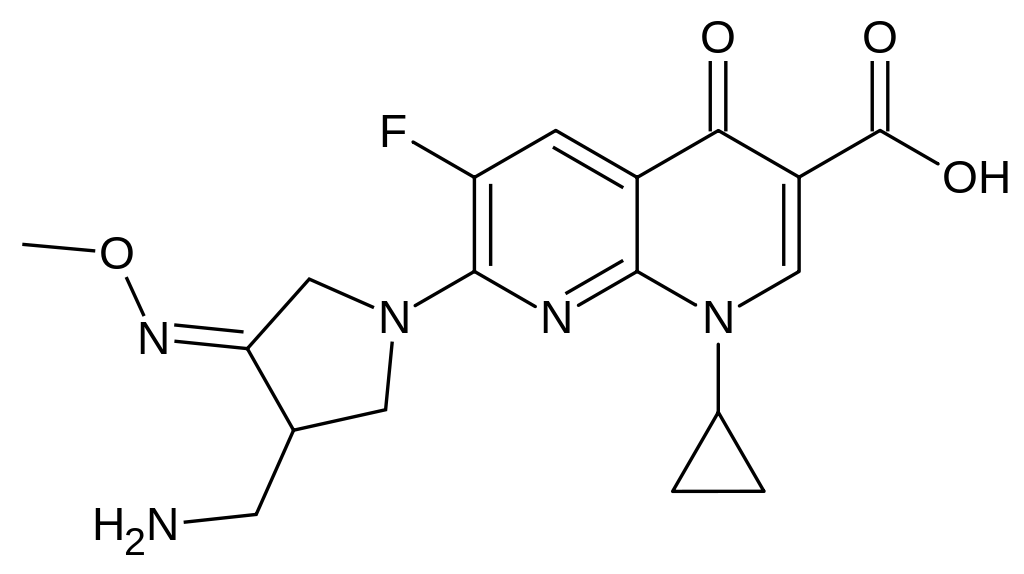
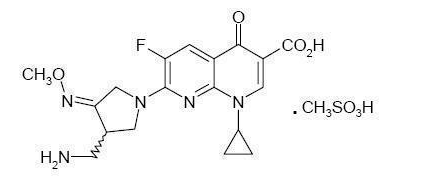
- FACTIVE (gemifloxacin mesylate) is a synthetic broad-spectrum antibacterial agent for oral administration. Gemifloxacin, a compound related to the fluoroquinolone class of antibiotics, is available as the mesylate salt in the sesquihydrate form. Chemically, gemifloxacin is (R,S)-7-[(4Z)-3-(aminomethyl)-4-(methoxyimino)-1-pyrrolidinyl]-1-cyclopropyl-6-fluoro-1,4-dihydro-4-oxo-1,8-naphthyridine-3-carboxylic acid.
- The mesylate salt is a white to light brown solid with a molecular weight of 485.49. Gemifloxacin is considered freely soluble at neutral pH (350 μg/mL at 37ºC, pH 7.0). Its empirical formula is C18H20FN5O4•CH4O3S and its chemical structure is:
- Each white to off-white, oval, film-coated FACTIVE tablet has breaklines and GE 320 debossed on both faces and contains gemifloxacin mesylate equivalent to 320 mg gemifloxacin. The inactive ingredients are crospovidone, hydroxypropyl methylcellulose, magnesium stearate, microcrystalline cellulose, polyethylene glycol, povidone, and titanium dioxide.
Pharmacodynamics
There is limited information regarding Pharmacodynamics of Gemifloxacin mesylate in the drug label.
Pharmacokinetics
Pharmacokinetics
- The pharmacokinetics of gemifloxacin are approximately linear over the dose range from 40 mg to 640 mg. There was minimal accumulation of gemifloxacin following multiple oral doses up to 640 mg a day for 7 days (mean accumulation <20%). Following repeat oral administration of 320 mg gemifloxacin once daily, steady-state is achieved by the third day of dosing.
Absorption and Bioavailability
- Gemifloxacin, given as an oral tablet, is rapidly absorbed from the gastrointestinal tract. Peak plasma concentrations of gemifloxacin were observed between 0.5 and 2 hours following oral tablet administration and the absolute bioavailability of the 320 mg tablet averaged approximately 71% (95% CI 60%-84%). Following repeat oral doses of 320 mg to healthy subjects, the mean ± SD maximal gemifloxacin plasma concentrations (Cmax) and systemic drug exposure (AUC (0-24)) were 1.61 ± 0.51 μg/mL (range 0.70-2.62 μg/mL) and 9.93 ± 3.07 μg•hr/mL (range 4.71-20.1 μg•hr/mL), respectively. In patients with respiratory and urinary tract infections (n=1423), similar estimates of systemic drug exposure were determined using a population pharmacokinetics analysis (geometric mean AUC (0-24), 8.36 μg•hr/mL; range 3.2 – 47.7 μg•hr/mL).
- The pharmacokinetics of gemifloxacin were not significantly altered when a 320 mg dose was administered with a high-fat meal. Therefore FACTIVE tablets may be administered without regard to meals.
Distribution
- In vitro binding of gemifloxacin to plasma proteins in healthy subjects is approximately 60 to 70% and is concentration independent. After repeated doses, the in vivo plasma protein binding in healthy elderly and young subjects ranged from 55% to 73% and was unaffected by age. Renal impairment does not significantly affect the protein binding of gemifloxacin. The blood-to-plasma concentration ratio of gemifloxacin was 1.2:1. The geometric mean for Vdss/F is 4.18 L/kg (range, 1.66 – 12.12 L/kg).
- Gemifloxacin is widely distributed throughout the body after oral administration. Concentrations of gemifloxacin in bronchoalveolar lavage fluid exceed those in the plasma. Gemifloxacin penetrates well into lung tissue and fluids. After five daily doses of 320 mg gemifloxacin, concentrations in plasma, bronchoalveolar macrophages, epithelial lining fluid and bronchial mucosa at approximately 2 hours were as in Table 1.

Metabolism
- Gemifloxacin is metabolized to a limited extent by the liver. The unchanged compound is the predominant drug-related component detected in plasma (approximately 65%) up to 4 hours after dosing. All metabolites formed are minor (<10% of the administered oral dose); the principal ones are N-acetyl gemifloxacin, the E-isomer of gemifloxacin and the carbamyl glucuronide of gemifloxacin. Cytochrome P450 enzymes do not play an important role in gemifloxacin metabolism, and the metabolic activity of these enzymes is not significantly inhibited by gemifloxacin.
Excretion
- Gemifloxacin and its metabolites are excreted via dual routes of excretion. Following oral administration of gemifloxacin to healthy subjects, a mean (± SD) of 61 ± 9.5% of the dose was excreted in the feces and 36 ± 9.3% in the urine as unchanged drug and metabolites. The mean (± SD) renal clearance following repeat doses of 320 mg was approximately 11.6 ± 3.9 L/hr (range 4.6-17.6 L/hr), which indicates active secretion is involved in the renal excretion of gemifloxacin. The mean (± SD) plasma elimination half-life at steady state following 320 mg to healthy subjects was approximately 7 ± 2 hours (range 4-12 hours).
Special Populations
- Pediatric: The pharmacokinetics of gemifloxacin in pediatric subjects have not been studied.
- Geriatric: In adult subjects, the pharmacokinetics of gemifloxacin are not affected by age.
- Gender: There are no significant differences between gemifloxacin pharmacokinetics in males and females when differences in body weight are taken into account. Population pharmacokinetic studies indicated that following administration of 320 mg gemifloxacin, AUC values were approximately 10% higher in healthy female patients compared to males. Males and females had mean AUC values of 7.98 μg•hr/mL (range, 3.21 – 42.71 μg•hr/mL) and 8.80 μg•hr/mL (range, 3.33 – 47.73 μg•hr/mL), respectively. No gemifloxacin dosage adjustment based on gender is necessary.
- Hepatic Insufficiency: The pharmacokinetics following a single 320 mg dose of gemifloxacin were studied in patients with mild (Child-Pugh Class A) to moderate (Child-Pugh Class B) liver disease. There was a mean increase in AUC (0-inf) of 34% and a mean increase in Cmax of 25% in these patients with hepatic impairment compared to healthy volunteers.
The pharmacokinetics of a single 320 mg dose of gemifloxacin were also studied in patients with severe hepatic impairment (Child-Pugh Class C). There was a mean increase in AUC (0-inf) of 45% and a mean increase in Cmax of 41% in these subjects with hepatic impairment compared to healthy volunteers.
These average pharmacokinetic increases are not considered to be clinically significant. There was no significant change in plasma elimination half-life in the mild, moderate or severe hepatic impairment patients. No dosage adjustment is recommended in patients with mild (Child-Pugh Class A), moderate (Child-Pugh Class B) or severe (Child-Pugh Class C) hepatic impairment.
- Renal Insufficiency: Results from population pharmacokinetic and clinical pharmacology studies with repeated 320 mg doses indicate the clearance of gemifloxacin is reduced and the plasma elimination is prolonged, leading to an average increase in AUC values of approximately 70% in patients with renal insufficiency. In the pharmacokinetic studies, gemifloxacin Cmax was not significantly altered in subjects with renal insufficiency. Dose adjustment in patients with creatinine clearance >40 mL/min is not required. Modification of the dosage is recommended for patients with creatinine clearance ≤40 mL/min.
Hemodialysis removes approximately 20 to 30% of an oral dose of gemifloxacin from plasma.
Photosensitivity Potential
- In a study of the skin response to ultraviolet and visible radiation conducted in 40 healthy volunteers, the minimum erythematous dose (MED) was assessed following administration of either gemifloxacin 160 mg once daily, gemifloxacin 320 mg once daily, ciprofloxacin 500 mg BID, or placebo for 7 days. At 5 of the 6 wavelengths tested (295-430 nm), the photosensitivity potential of gemifloxacin was not statistically different from placebo. At 365 nm (UVA region), gemifloxacin showed a photosensitivity potential similar to that of ciprofloxacin 500 mg BID and the photosensitivity potential for both drugs were statistically greater than that of placebo. Photosensitivity reactions were reported rarely in clinical trials with gemifloxacin (0.039%).
- It is difficult to ascribe relative photosensitivity/phototoxicity among various fluoroquinolones during actual patient use because other factors play a role in determining a subject’s susceptibility to this adverse event such as: a patient’s skin pigmentation, frequency and duration of sun and artificial ultraviolet light (UV) exposure, wearing of sun screen and protective clothing, the use of other concomitant drugs and the dosage and duration of fluoroquinolone therapy.
Drug-Drug Interactions
Antacids/Di- and Trivalent Cations: The systemic availability of gemifloxacin is significantly reduced when an aluminum- and magnesium- containing antacid is concomitantly administered (AUC decreased 85%; Cmax decreased 87%). Administration of an aluminum- and magnesium- containing antacid or ferrous sulfate (325 mg) at 3 hours before or at 2 hours after gemifloxacin did not significantly alter the systemic availability of gemifloxacin. Therefore, aluminum- and/or magnesium- containing antacids, ferrous sulfate (iron), multivitamin preparations containing zinc or other metal cations, or Videx® (didanosine) chewable/buffered tablets or the pediatric powder for oral solution should not be taken within 3 hours before or 2 hours after taking FACTIVE tablets.
Calcium carbonate (1000 mg) given either 2 hr before or 2 hr after gemifloxacin administration showed no notable reduction in gemifloxacin systemic availability. Calcium carbonate administered simultaneously with gemifloxacin resulted in a small, not clinically significant, decrease in gemifloxacin exposure [AUC (0-inf) decreased 21% and Cmax decreased].
Sucralfate: When sucralfate (2 g) was administered 3 hours prior to gemifloxacin, the oral bioavailability of gemifloxacin was significantly reduced (53% decrease in AUC; 69% decrease in Cmax). When sucralfate (2 g) was administered 2 hours after gemifloxacin, the oral bioavailability of gemifloxacin was not significantly affected; therefore FACTIVE should be taken at least 2 hours before sucralfate.
In Vitro Metabolism: Results of in vitro inhibition studies indicate that hepatic cytochrome P450 (CYP450) enzymes do not play an important role in gemifloxacin metabolism. Therefore gemifloxacin should not cause significant in vivo pharmacokinetic interactions with other drugs that are metabolized by CYP450 enzymes.
Theophylline: Gemifloxacin 320 mg at steady-state did not affect the repeat dose pharmacokinetics of theophylline (300 to 400 mg BID to healthy male subjects).
Digoxin: Gemifloxacin 320 mg at steady-state did not affect the repeat dose pharmacokinetics of digoxin (0.25 mg once daily to healthy elderly subjects).
Oral Contraceptives: The effect of an oral estrogen/progesterone contraceptive product (once daily for 21 days) on the pharmacokinetics of gemifloxacin (320 mg once daily for 6 days) in healthy female subjects indicates that concomitant administration caused an average reduction in gemifloxacin AUC and Cmax of 19% and 12%. These changes are not considered clinically significant. Gemifloxacin 320 mg at steady-state did not affect the repeat dose pharmacokinetics of an ethinylestradiol/levonorgestrol oral contraceptive product (30 μg/150 μg once daily for 21 days to healthy female subjects).
Cimetidine: Co-administration of a single dose of 320 mg gemifloxacin with cimetidine 400 mg four times daily for 7 days resulted in slight average increases in gemifloxacin AUC(0-inf) and Cmax of 10% and 6%, respectively. These increases are not considered clinically significant.
Omeprazole: Co-administration of a single dose of 320 mg gemifloxacin with omeprazole 40 mg once daily for 4 days resulted in slight average increases in gemifloxacin AUC(0-inf) and Cmax of 10% and 11%, respectively. These increases are not considered clinically significant.
Warfarin: Administration of repeated doses of gemifloxacin (320 mg once daily for 7 days) to healthy subjects on stable warfarin therapy had no significant effect on warfarin-induced anticoagulant activity (i.e., International Normalized Ratios for Prothrombin Time).
Probenecid: Administration of a single dose of 320 mg gemifloxacin to healthy subjects who also received repeat doses of probenecid (total dose = 4.5 g) reduced the mean renal clearance of gemifloxacin by approximately 50%, resulting in a mean increase of 45% in gemifloxacin AUC (0-inf) and a prolongation of mean half-life by 1.6 hours. Mean gemifloxacin Cmax increased 8%.
Nonclinical Toxicology
There is limited information regarding Nonclinical Toxicology of Gemifloxacin mesylate in the drug label.
Clinical Studies
Acute Bacterial Exacerbation of Chronic Bronchitis (ABECB)
- Gemifloxacin mesylate (320 mg once daily for 5 days) was evaluated for the treatment of acute bacterial exacerbation of chronic bronchitis in three pivotal double-blind, randomized, actively-controlled clinical trials (studies 068, 070, and 212). The primary efficacy parameter in these studies was the clinical response at follow-up (day 13 to 24). The results of the clinical response at follow-up for the principal ABECB studies demonstrate that FACTIVE 320 mg PO once daily for 5 days was at least as good as the comparators given for 7 days. The results are shown in Table 6 below.

Community Acquired Pneumonia (CAP)
5 Day Treatment Regimen
- To evaluate the safety and efficacy of a 5-day course of gemifloxacin mesylate, 510 outpatient and hospitalized adults with clinically and radiologically determined mild to moderate community-acquired pneumonia were clinically evaluated in a double-blind, randomized, prospective, multicenter study comparing gemifloxacin mesylate 320 mg for five days to gemifloxacin mesylate 320 mg for seven days (Study OP-634-001).
- Clinical success rates in the clinically evaluable population were 95.0% in the 5 day group and 92.1% in the 7 day group.

The microbiological efficacy of the 5-day regimen was documented for pathogens listed in Table 8 below.

7 Day Treatment Regimen
- Previous clinical studies evaluated the efficacy of gemifloxacin mesylate in a 7-day treatment of CAP in adults. This clinical program consisted of three double-blind, randomized, actively-controlled clinical studies (studies 011, 012, and 049) and one open-label, actively-controlled study (study 185). In addition, two uncontrolled studies (studies 061 and 287) were conducted. Three of the studies, controlled study 011 and the uncontrolled studies, had a fixed 7-day duration of treatment for gemifloxacin mesylate. Controlled study 011 compared a 7-day course of gemifloxacin mesylate with a 10-day treatment course of amoxicillin/clavulanate (1g/125 mg TID) and clinical success rates were similar between treatment arms. The results of comparative studies 049, 185, and 012 were supportive although treatment duration could have been 7 to 14 days. The results of the clinical studies with a fixed 7-day duration of gemifloxacin mesylate are shown in Table 9.

For uncontrolled studies, the 95% CI around the success rate is shown
- The combined bacterial eradication rates for patients treated with a fixed 7-day treatment regimen of FACTIVE are shown in Table 10.

7 Day Treatment Regimen of Community-Acquired Pneumonia Due to Multi-Drug Resistant Streptococcus pneumoniae (MDRSP)
- Gemifloxacin mesylate was also effective in the treatment of CAP due to multi-drug resistant Streptococcus pneumoniae (MDRSP*). Of 35 patients with MDRSP treated for 7 days, 29 (82.9%) achieved clinical and bacteriological success at follow-up. The clinical and bacteriological success for the 35 patients with MDRSP isolates are shown in Table 11.
- MDRSP: multi-drug resistant Streptococcus pneumoniae, includes isolates previously known as PRSP (penicillin-resistant Streptococcus pneumoniae), and are strains resistant to two or more of the following antibiotics: penicillin (MIC ≥2 μg/mL), 2nd generation cephalosporins (e.g., cefuroxime), macrolides, tetracyclines and trimethoprim/sulfamethoxazole.

- Not all isolates were resistant to all antimicrobial classes tested. Success and eradication rates are summarized in Table 12 below.

Clinical Safety Study of Rash
- To further characterize gemifloxacin-associated rash, which in early clinical studies appeared to be associated with age less than 40 and female gender, a clinical pharmacology study was conducted. The study enrolled 1,011 healthy female volunteers less than 40 years of age. Subjects were randomized in a 5:1 ratio to receive either FACTIVE 320 mg PO daily (819 subjects) or ciprofloxacin 500 mg PO twice daily for 10 days (164 subjects). This study was designed to enroll subjects at a high risk for rash (women <40 years of age and dosing beyond the recommended duration of therapy for gemifloxacin mesylate [10 days]) and over estimates the risk to patients taking gemifloxacin mesylate as prescribed. Subjects who received gemifloxacin mesylate were 7 times more likely to develop rash than those who received ciprofloxacin. Of the 260 rashes in subjects receiving gemifloxacin mesylate, the majority of rashes were maculopapular and of mild to moderate severity; 7% of the rashes were reported as severe, and severity appeared to correlate with the extent of the rash. In 68% of the subjects reporting a severe rash and approximately 25% of all those reporting rash, >60% of the body surface area was involved; the characteristics of the rash were otherwise indistinguishable from those subjects reporting a mild rash. The histopathology was consistent with the clinical observation of uncomplicated exanthematous morbilliform eruption. Approximately 11% of the rashes were described as being “urticaria-like”. There were no documented cases of hypersensitivity syndrome or findings suggestive of angioedema or other serious cutaneous reactions.
- The majority of rashes (81.9%) occurred on days 8 through 10 of the planned 10 day course of gemifloxacin mesylate; 2.7% of rash events occurred within one day of the start of dosing. The median duration of rash was 6 days. The rash resolved without treatment in the majority of subjects. Approximately 19% received antihistamines and 5% received steroids, although the therapeutic benefit of these therapies is uncertain.
In the second part of this study after a 4 to 6 week wash out period, subjects developing a rash on gemifloxacin mesylate were treated with ciprofloxacin (n=136) or placebo (n=50); 5.9% developed rash when treated with ciprofloxacin and 2.0% developed rash when treated with placebo. The cross sensitization rate to other fluoroquinolones was not evaluated in this clinical study. There was no evidence of sub-clinical sensitization to gemifloxacin mesylate on a second exposure (i.e., subjects who had not developed a rash to gemifloxacin mesylate in the first part of the study were not at higher risk of developing a rash to gemifloxacin mesylate with a second exposure).
- There was no relationship between the incidence of rash and systemic exposure (Cmax and AUC) to either gemifloxacin or its major metabolite, N-acetyl gemifloxacin.
How Supplied
- FACTIVE (gemifloxacin mesylate) is available as white to off-white, oval, film-coated tablets with breaklines and GE 320 debossed on both faces. Each tablet contains gemifloxacin mesylate equivalent to 320 mg of gemifloxacin.
320 mg Unit of Use (CR*) 5's NDC 44004-321-05 320 mg Unit of Use (CR*) 7's NDC 44004-321-07
- Child Resistant
Storage
- Store at 25ºC (77ºF); excursions permitted to 15º-30ºC (59º-86ºF) [see USP Controlled Room Temperature]. Protect from light.
Images
Drug Images
{{#ask: Page Name::Gemifloxacin mesylate |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel


{{#ask: Label Page::Gemifloxacin mesylate |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
There is limited information regarding Patient Counseling Information of Gemifloxacin mesylate in the drug label.
Precautions with Alcohol
- Alcohol-Gemifloxacin mesylate interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
- FACTIVE
Look-Alike Drug Names
- A® — B®[1]
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
- ↑ "http://www.ismp.org". External link in
|title=(help)
{{#subobject:
|Page Name=Gemifloxacin mesylate
|Pill Name=No image.jpg
|Drug Name=
|Pill Ingred=|+sep=;
|Pill Imprint=
|Pill Dosage={{{dosageValue}}} {{{dosageUnit}}}
|Pill Color=|+sep=;
|Pill Shape=
|Pill Size (mm)=
|Pill Scoring=
|Pill Image=
|Drug Author=
|NDC=
}}
{{#subobject:
|Label Page=Gemifloxacin mesylate |Label Name=Gemifloxacin image1.jpg
}}
{{#subobject:
|Label Page=Gemifloxacin mesylate |Label Name=Gemifloxacin image.jpg
}}
{{#subobject:
|Label Page=Gemifloxacin mesylate |Label Name=Gemifloxacin ingredients and appearance.png
}}