Cortisol
…
 | |
 | |
| Clinical data | |
|---|---|
| Pregnancy category |
|
| Routes of administration | Oral tablets, intravenously, topical |
| ATC code | |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| E number | {{#property:P628}} |
| ECHA InfoCard | {{#property:P2566}}Lua error in Module:EditAtWikidata at line 36: attempt to index field 'wikibase' (a nil value). |
| Chemical and physical data | |
| Formula | C21H30O5 |
| Molar mass | 362.465 |
|
WikiDoc Resources for Cortisol |
|
Articles |
|---|
|
Most recent articles on Cortisol |
|
Media |
|
Evidence Based Medicine |
|
Clinical Trials |
|
Ongoing Trials on Cortisol at Clinical Trials.gov Clinical Trials on Cortisol at Google
|
|
Guidelines / Policies / Govt |
|
US National Guidelines Clearinghouse on Cortisol
|
|
Books |
|
News |
|
Commentary |
|
Definitions |
|
Patient Resources / Community |
|
Directions to Hospitals Treating Cortisol Risk calculators and risk factors for Cortisol
|
|
Healthcare Provider Resources |
|
Causes & Risk Factors for Cortisol |
|
Continuing Medical Education (CME) |
|
International |
|
|
|
Business |
|
Experimental / Informatics |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1] Phone:617-632-7753
Cortisol is a corticosteroid hormone produced by the adrenal cortex (in the adrenal gland). It is a vital hormone that is often referred to as the "stress hormone" as it is involved in the response to stress. It increases blood pressure, blood sugar levels and has an immunosuppressive action. In pharmacology, the synthetic form of cortisol is referred to as hydrocortisone, and is used to treat allergies and inflammation as well as cortisol production deficiencies. When first introduced as a treatment for rheumatoid arthritis, it was referred to as Compound E.
Physiology
Diurnal variation
The amount of cortisol present in the serum undergoes diurnal variation, with the highest levels present in the early morning, and the lowest levels present around midnight, 3-5 hours after the onset of sleep. Information about the light/dark cycle is transmitted from the retina to the paired suprachiasmatic nuclei in the hypothalamus. The pattern is not present at birth (estimates of when it starts vary from two weeks to 9 months.[1])
Changed patterns of serum cortisol levels have been observed in connection with abnormal ACTH levels, clinical depression, psychological stress, and such physiological stressors as hypoglycemia, illness, fever, trauma, surgery, fear, pain, physical exertion or extremes of temperature.
There is also significant individual variation, although a given person tends to have consistent rhythms.
Effects
In normal release, cortisol (like other glucocorticoid agents) has widespread actions which help restore homeostasis after stress. (These normal endogenous functions are the basis for the physiological consequences of chronic stress - prolonged cortisol secretion.)
- It acts as a physiological antagonist to insulin by promoting glycogenolysis (breakdown of glycogen), breakdown of lipids (lipolysis), and proteins, and mobilization of extrahepatic amino acids and ketone bodies. This leads to increased circulating glucose concentrations (in the blood). There is a decreased glycogen formation in the liver . [2] Prolonged cortisol secretion causes hyperglycemia.
- It can weaken the activity of the immune system . Cortisol prevents proliferation of T-cells by rendering the interleukin-2 producer T-cells unresponsive to interleukin-1 (IL-1), and unable to produce the T-cell growth factor.[3] It reflects leukocyte redistribution to lymph nodes, bone marrow, and skin. Acute administration of corticosterone (the endogenous Type I and Type II receptor agonist), or RU28362 (a specific Type II receptor agonist), to adrenalectomized animals induced changes in leukocyte distribution.
- It lowers bone formation thus favoring development of osteoporosis in the long term. Cortisol moves potassium into cells in exchange for an equal number of sodium ions.[4] This can cause a major problem with the hyperkalemia of metabolic shock from surgery.
- It may help to create memories when exposure is short-term; this is the proposed mechanism for storage of flash bulb memories. However, long-term exposure to cortisol results in damage to cells in the hippocampus. This damage results in impaired learning.
- It increases blood pressure by increasing the sensitivity of the vasculature to epinephrine and norepinephrine. In the absence of cortisol, widespread vasodilation occurs.
- It inhibits the secretion of corticotropin-releasing hormone (CRH), resulting in feedback inhibition of ACTH secretion. Some researchers believe that this normal feedback system may break down when animals are exposed to chronic stress.
- It increases the effectiveness of catecholamines.
- It allows for the kidneys to produce hypotonic urine.
- It has anti-inflammatory effects by reducing histamine secretion and stabilizing lysosomal membranes. The stabilization of lysosomal membranes prevents their rupture, thereby preventing damage to healthy tissues.
- It stimulates hepatic detoxification by inducing tryptophan oxygenase (to reduce serotonin levels in the brain), glutamine synthase (reduce glutamate and ammonia levels in the brain), cytochrome P-450 hemoprotein (mobilizes arachidonic acid), and metallothionein (reduces heavy metals in the body).
In addition to the effects caused by cortisol binding to the glucocorticoid receptor, because of its molecular similarity to aldosterone, it also binds to the mineralocorticoid receptor. (It binds with less affinity to it than aldosterone does, but the concentration of blood cortisol is higher than that of blood aldosterone.)
Binding
Most serum cortisol, all but about 4%, is bound to proteins including corticosteroid binding globulin (CBG), and serum albumin. Only free cortisol is available to most receptors.
Diseases and disorders
- Hypercortisolism: Excessive levels of cortisol in the blood result in Cushing's syndrome.
- Hypocortisolism, or adrenal insufficiency: If on the other hand the adrenal glands do not produce sufficient amounts of cortisol, Addison's disease is the consequence.
The relationship between cortisol and ACTH is as follows:
| Plasma Cortisol | Plasma ACTH | |
|---|---|---|
| Primary Hypercortisolism (Cushing's syndrome) | ↑ | ↓ |
| Secondary Hypercortisolism (pituitary, Cushing's disease) | ↑ | ↑ |
| Primary Hypocortisolism (Addison's disease) | ↓ | ↑ |
| Secondary Hypocortisolism (pituitary) | ↓ | ↓ |
Pharmacology
Hydrocortisone is the chemical form of cortisol used for oral administration or intravenous injection. It is used as an immunosuppressive drug, given by injection in the treatment of severe allergic reactions such as anaphylaxis and angioedema, in place of prednisolone in patients who need steroid treatment but cannot take oral medication, and peri-operatively in patients on long-term steroid treatment to prevent an Addisonian crisis.
It is given by topical application for its anti-inflammatory effect in allergic rashes, eczema, psoriasis and certain other inflammatory conditions.
It may also be injected into inflamed joints resulting from diseases such as gout.
Compared to prednisolone, hydrocortisone is about 1/4th the strength (for the anti-inflammatory effect only). Dexamethasone is about 40 times stronger than hydrocortisone. For side effects, see corticosteroid and prednisolone. Non prescription 1% hydrocortisone cream or ointment are available; stronger forms are prescription only.[2]
Biochemistry
Biosynthesis
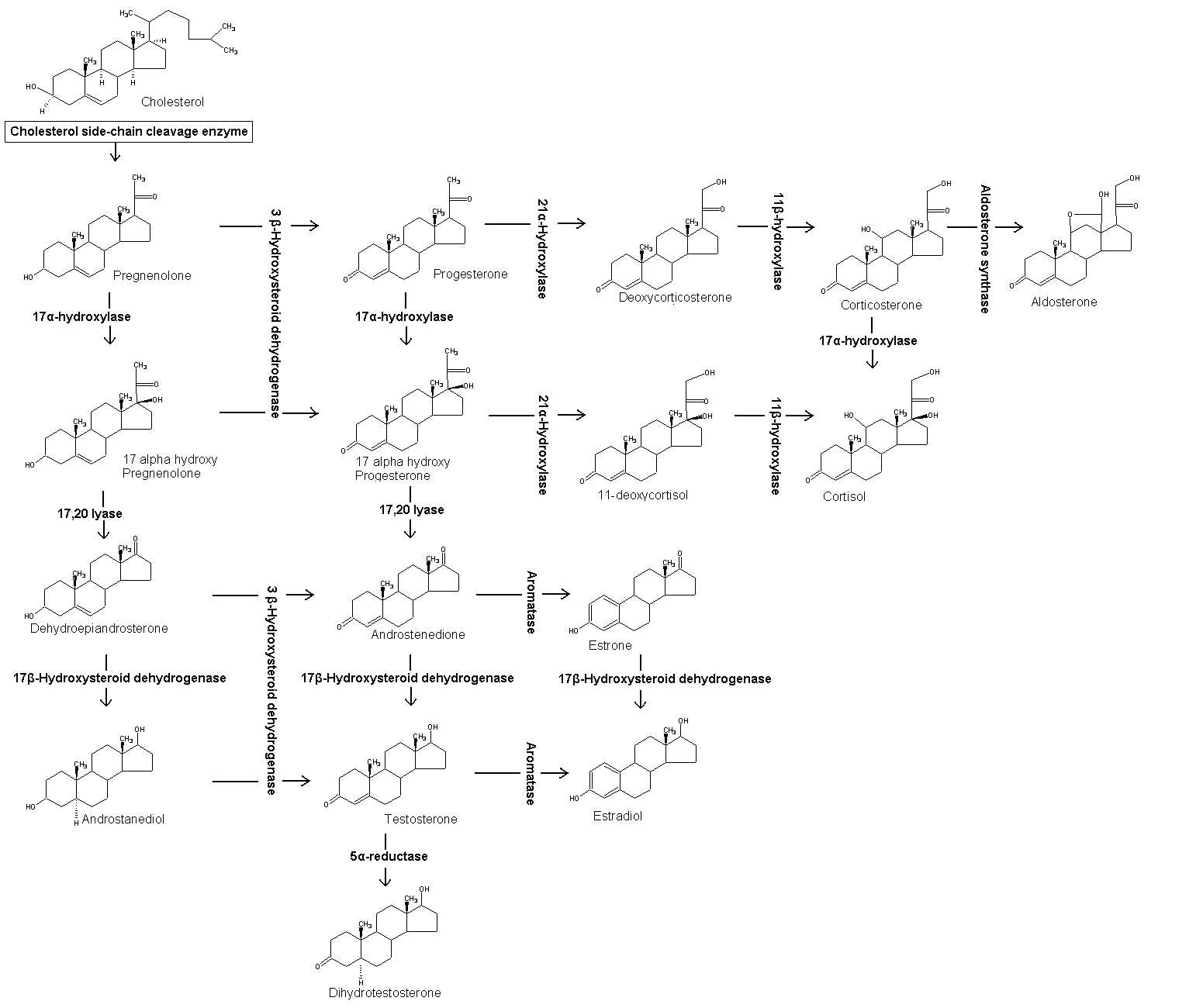
Cortisol is synthesized from cholesterol. The synthesis takes place in the zona fasciculata of the cortex of the adrenal glands. (The name cortisol comes from cortex.) While the adrenal cortex also produces aldosterone (in the zona glomerulosa) and some sex hormones (in the zona reticularis), cortisol is its main secretion. The medulla of the adrenal gland lies under the cortex and mainly secretes the catecholamines, adrenaline (epinephrine) and noradrenaline (norepinephrine) under sympathetic stimulation (more epinephrine is produced than norepinephrine, in a ratio 4:1).
The synthesis of cortisol in the adrenal gland is stimulated by the anterior lobe of the pituitary gland with adrenocorticotropic hormone (ACTH); production of ACTH is in turn stimulated by corticotropin-releasing hormone (CRH), released by the hypothalamus. ACTH increases the concentration of cholesterol in the inner mitochondrial membrane (via regulation of STAR (steroidogenic acute regulatory) protein). The cholesterol is converted to pregnenolone, catalysed by Cytochrome P450SCC (side chain cleavage).
Metabolism
Cortisol is metabolized by the 11-beta hydroxysteroid dehydrogenase system (11-beta HSD), which consists of two enzymes: 11-beta HSD1 and 11-beta HSD2.
- 11-beta HSD1 utilizes the cofactor NADPH to convert biologically inert cortisone to biologically active cortisol.
- 11-beta HSD2 utilizes the cofactor NAD+ to convert cortisol to cortisone.
Overall the net effect is that 11-beta HSD1 serves to increase the local concentrations of biologically active cortisol in a given tissue, while 11-beta HSD2 serves to decrease the local concentrations of biologically active cortisol.
An alteration in 11-beta HSD1 has been suggested to play a role in the pathogenesis of obesity, hypertension, and insulin resistance, sometimes referred to the metabolic syndrome.
An alteration in 11-beta HSD2 has been implicated in essential hypertension and is known to lead to the syndrome of apparent mineralocorticoid excess (SAME).
See also
- Cushing's syndrome
- HPA axis
- Hypopituitarism
- Post-traumatic stress disorder
- Central serous retinopathy
- CortiSlim
- Relacore, a pill which claims to reduce Cortisol.
Additional images
References
- ↑ de Weerth C, Zijl R, Buitelaar J (2003). "Development of cortisol circadian rhythm in infancy". Early Hum Dev. 73 (1–2): 39–52. PMID 12932892.
- ↑ Freeman, Scott (2002). Biological Science. Prentice Hall; 2nd Pkg edition (December 30, 2004). ISBN 0-13-218746-9.
- ↑ Palacios R., Sugawara I. (1982). "Hydrocortisone abrogates proliferation of T cells in autologous mixed lymphocyte reaction by rendering the interleukin-2 Producer T cells unresponsive to interleukin-1 and unable to synthesize the T-cell growth factor". Scand J Immunol. 15 (1): 25–31. PMID 6461917.
- ↑ Knight, R.P., Jr. Kornfield, D.S. Glaser, G.H. Bondy, P.K. (1955). "Effects of intravenous hydrocortisone on electrolytes of serum and urine in man". J Clin Endocrinol Metab. 15 (2): 176–81. PMID 13233328.
External links
Template:Corticosteroids Template:Antidiarrheals, intestinal anti-inflammatory/anti-infective agents Template:Otologicals
de:Cortisol eo:Kortisolo he:קורטיזול nl:Cortisol no:Kortisol sl:Kortizol fi:Kortisoli sv:Kortisol
- Pages with script errors
- CS1 maint: Multiple names: authors list
- E number from Wikidata
- ECHA InfoCard ID from Wikidata
- Articles without EBI source
- Chemical pages without ChemSpiderID
- Chemical pages without DrugBank identifier
- Articles without KEGG source
- Articles without InChI source
- Articles without UNII source
- Drugs with no legal status
- Articles containing unverified chemical infoboxes
- Glucocorticoids
- Immunosuppressive agents
- Endocrinology