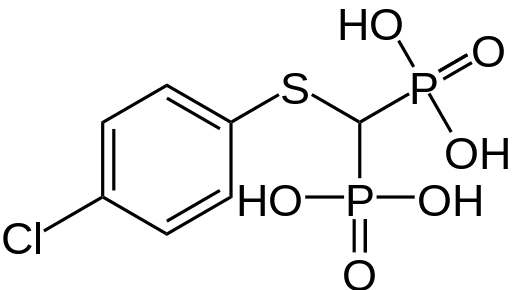
Tiludronate
 | |
| Clinical data | |
|---|---|
| Trade names | Skelid |
| AHFS/Drugs.com | International Drug Names |
| ATC code | |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| E number | {{#property:P628}} |
| ECHA InfoCard | {{#property:P2566}}Lua error in Module:EditAtWikidata at line 36: attempt to index field 'wikibase' (a nil value). |
| Chemical and physical data | |
| Formula | C7H9ClO6P2S |
| Molar mass | 318.609 g/mol |
| 3D model (JSmol) | |
| |
| |
| | |
|
WikiDoc Resources for Tiludronate |
|
Articles |
|---|
|
Most recent articles on Tiludronate Most cited articles on Tiludronate |
|
Media |
|
Powerpoint slides on Tiludronate |
|
Evidence Based Medicine |
|
Clinical Trials |
|
Ongoing Trials on Tiludronate at Clinical Trials.gov Clinical Trials on Tiludronate at Google
|
|
Guidelines / Policies / Govt |
|
US National Guidelines Clearinghouse on Tiludronate
|
|
Books |
|
News |
|
Commentary |
|
Definitions |
|
Patient Resources / Community |
|
Patient resources on Tiludronate Discussion groups on Tiludronate Patient Handouts on Tiludronate Directions to Hospitals Treating Tiludronate Risk calculators and risk factors for Tiludronate
|
|
Healthcare Provider Resources |
|
Causes & Risk Factors for Tiludronate |
|
Continuing Medical Education (CME) |
|
International |
|
|
|
Business |
|
Experimental / Informatics |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]
Overview
Tiludronic acid (INN; also known as tiludronate) is a bisphosphonate used for treatment of Paget's disease of bone (osteitis deformans) in human medicine. It has the tradename Skelid. In veterinary medicine, tiludronic acid is used to treat navicular disease and bone spavin in horses. Its tradenames are Tildren and Equidronate. It is approved for treatment of navicular disease and distal tarsal osteoarthritis in Europe,[1] and was approved for treatment of navicular disease in the United States in 2014.
Mechanism of action
Tiludronate is a non-nitrogenous bisphosphonate that inhibits osteoclasts, the primary cell responsible for the breakdown of bone required for bone remodeling. Non-nitrogenous bisphosphonates are metabolized by osteoclasts to compounds that then replace a portion of the adenosine triphosphate (ATP) molecule, making it non-functional. These non-functional molecules then competitively inhibit ATP in the cell, reducing cell energy and leading to apoptosis.[2] Decreased levels of osteoclasts subsequently reduce the degree of breakdown of bone and bone turnover. Non-nitrogenous bisphosphonates are less potent relative to nitrogenous bisphosphonates.
Use in equine medicine
Tiludronate has been used primarily for the treatment of diseases in horses that are associated with inappropriate osteolysis, such as navicular disease[3] and osteoarthritis. It has specifically been shown to improve lameness in horses with osteoarthritis of the distal hock joints (bone spavin)[4] and vertebral column.[5]
Method of administration
Tildren is administered intravenously. It is labeled for 0.1mg/kg dosing, once daily for 10 days by slow intravenous injection, which for a 500kg horse works out to be 1 vial per day. However, one study giving all 10 doses at once (1 mg/kg IV as a single CRI) was found to have the same pharmacological effects, and is used clinically.[6] It may be given systemically or locally, by regional limb perfusion. Although RLP is thought to have certain benefits, including decreased cost and reduced risk of adverse effects, some diseases must be treated systemically, such as osteoarthritis of the vertebral column. Systemic administration is often given by adding a 1 mg/kg dose into a 1 liter fluid bag, which is slowly given over 60-90 minutes. Its effects are reported to last 4 months or longer, with a peak effect 6-8 weeks post treatment.[7] The effects of regional limb perfusion has come into question due to in vitro studies showing that high doses given by RLP or intraarticular injection may damage articular cartilage by chondrocyte apoptosis.[8] Further studies are needed to evaluate the safety of Tildren administration via RLP.
Adverse reactions and contraindications
Tildren has been shown to have several adverse effects.[9]
- Signs of colic, which is usually self-limiting, occurs in 30-45% of horses.
- Tachycardia
- Electrolyte disturbances: primarily calcium, magnesium, and potassium, which can last for several hours. Caution should be used in horses with disease processes that could be affected by electrolyte disturbances, such as hyperkalemic periodic paralysis or cardiac disease.
- Kidney damage: it is eliminated by the kidney and is not recommended for use in animals with impaired renal function.
- Less serious reactions include stiffness of the neck, decreased appetite, fever, and increased urination.
It is not recommended for under four years of age due to lack of studies evaluating its safety in growing animals, or pregnant or lactating animals since its affect on fetal one has not been studied.
References
- ↑ Kamm L, McIlwraith W, Kawcak C. A review of the Efficacy of Tiludronate in the Horse. Journal or Equine Veterinary Science. Vol 28(4): 209-214.
- ↑ Rogers MJ, Crockett JC, Coxon FP, Monkkonen J. 2011. Biochemical and molecular mechanisms of action of bisphosphonates. Bone 49:34-41
- ↑ DENOIX, J.M., THIBAUD, D. and RICCIO, B. (2003), Tiludronate as a new therapeutic agent in the treatment of navicular disease: a double-blind placebo-controlled clinical trial. Equine Veterinary Journal, 35: 407–413. doi: 10.2746/042516403776014226
- ↑ GOUGH, M. R., THIBAUD, D. and SMITH, R. K. W. (2010), Tiludronate infusion in the treatment of bone spavin: A double blind placebo-controlled trial. Equine Veterinary Journal, 42: 381–387. doi: 10.1111/j.2042-3306.2010.00120
- ↑ V. Coudry, D. Thibaud; B. Riccio; F. Audigié; D. Didierlaurent; M Denoix. Efficacy of tiludronate in the treatment of horses with signs of pain associated with osteoarthritic lesions of the thoracolumbar vertebral column. American Journal of Veterinary Research. Vol. 68, No. 3, Pages 329-337 March 2007
- ↑ DELGUSTE, C., AMORY, H., GUYONNET, J., THIBAUD, D., GARNERO, P., DETILLEUX, J., LEPAGE, O. M. and DOUCET, M. (2008), Comparative pharmacokinetics of two intravenous administration regimens of tiludronate in healthy adult horses and effects on the bone resorption marker CTX-1. Journal of Veterinary Pharmacology and Therapeutics, 31: 108–116. doi: 10.1111/j.1365-2885.2007.00936
- ↑ Allen KA, Johns S, Hyman SS, Sislak MD, Davis S, Amory J. How to Diagnose and Treat Back Pain in the Horse. Proc. AAEP. Vol 56: 384-388
- ↑ Duesterdieck-Zellmer KF, Driscoll N, Ott JF. Concentration-dependent effects of tiludronate on equine articular cartilage explants incubated with and without interleukin-1β. American Journal of Veterinary Research. Oct 2012, Vol. 73, No. 10, Pages 1530-1539. doi: 10.2460/ajvr.73.10.1530
- ↑ U.S. Food and Drug Administration. "FDA Provides Equine Veterinarians with Important Information about TILDREN and OSPHOS for Navicular Syndrome in Horses". http://www.fda.gov/AnimalVeterinary/ResourcesforYou/ucm406581.htm. External link in
|website=(help); Missing or empty|url=(help);|access-date=requires|url=(help)
External links
- Pages with script errors
- CS1 errors: external links
- Pages using web citations with no URL
- Pages using citations with accessdate and no URL
- Template:drugs.com link with non-standard subpage
- Articles with changed DrugBank identifier
- Articles with changed ChemSpider identifier
- Articles with changed KEGG identifier
- Articles with changed EBI identifier
- E number from Wikidata
- ECHA InfoCard ID from Wikidata
- Articles with changed InChI identifier
- Chemical articles with unknown parameter in Infobox drug
- Drugs with no legal status
- Drugboxes which contain changes to verified fields
- Drugboxes which contain changes to watched fields
- Bisphosphonates
- Drug