Axicabtagene ciloleucel
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Yashasvi Aryaputra[2], Anmol Pitliya, M.B.B.S. M.D.[3]
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Black Box Warning
|
CYTOKINE RELEASE SYNDROME and NEUROLOGIC TOXICITIES
See full prescribing information for complete Boxed Warning.
|
Overview
Axicabtagene ciloleucel is a CD19-directed genetically modified autologous T cell immunotherapy that is FDA approved for the treatment of adult patients with relapsed or refractory large B-cell lymphoma after two or more lines of systemic therapy, including diffuse large B-cell lymphoma (DLBCL) not otherwise specified, primary mediastinal large B-cell lymphoma, high grade B-cell lymphoma, and DLBCL arising from follicular lymphoma.. There is a Black Box Warning for this drug as shown here. Common adverse reactions include cytokine release syndrome, fever, hypotension, encephalopathy, tachycardia, fatigue, headache, decreased appetite, chills, diarrhea, febrile neutropenia, infections-pathogen unspecified, nausea, hypoxia, tremor, cough, vomiting, dizziness, constipation, and cardiac arrhythmias.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Indications:
- Axicabtagene ciloleucel is a CD19-directed genetically modified autologous T cell immunotherapy indicated for the treatment of adult patients with relapsed or refractory large B-cell lymphoma after two or more lines of systemic therapy, including diffuse large B-cell lymphoma (DLBCL) not otherwise specified, primary mediastinal large B-cell lymphoma, high grade B-cell lymphoma, and DLBCL arising from follicular lymphoma.
- Limitation of Use: axicabtagene ciloleucel is not indicated for the treatment of patients with primary central nervous system lymphoma.
Dose
- Each single infusion bag of axicabtagene ciloleucel contains a suspension of chimeric antigen receptor (CAR)-positive T cells in approximately 68 mL. The target dose is 2 × 106 CAR-positive viable T cells per kg body weight, with a maximum of 2 × 108 CAR-positive viable T cells.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding axicabtagene ciloleucel Off-Label Guideline-Supported Use and Dosage (Adult) in the drug label.
Non–Guideline-Supported Use
There is limited information regarding axicabtagene ciloleucel Off-Label Non-Guideline-Supported Use and Dosage (Adult) in the drug label.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
There is limited information regarding Axicabtagene ciloleucel FDA-Labeled Indications and Dosage (Pediatric) in the drug label.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding axicabtagene ciloleucel Off-Label Guideline-Supported Use and Dosage (Pediatric) in the drug label.
Non–Guideline-Supported Use
There is limited information regarding axicabtagene ciloleucel Off-Label Non-Guideline-Supported Use and Dosage (Pediatric) in the drug label.
Contraindications
- None
Warnings
|
CYTOKINE RELEASE SYNDROME and NEUROLOGIC TOXICITIES
See full prescribing information for complete Boxed Warning.
|
Cytokine Release Syndrome (CRS)
- CRS, including fatal or life-threatening reactions, occurred following treatment with axicabtagene ciloleucel. In Study 1, CRS occurred in 94% (101/108) of patients receiving axicabtagene ciloleucel, including ≥ Grade 3 (Lee grading system1) CRS in 13% (14/108) of patients. Among patients who died after receiving axicabtagene ciloleucel, four had ongoing CRS events at the time of death. The median time to onset was 2 days (range: 1 to 12 days) and the median duration of CRS was 7 days (range: 2 to 58 days). Key manifestations of CRS include fever (78%), hypotension (41%), tachycardia (28%), hypoxia (22%), and chills (20%). Serious events that may be associated with CRS include cardiac arrhythmias (including atrial fibrillation and ventricular tachycardia), cardiac arrest, cardiac failure, renal insufficiency, capillary leak syndrome, hypotension, hypoxia, and hemophagocytic lymphohistiocytosis/macrophage activation syndrome (HLH/MAS).
- Ensure that 2 doses of tocilizumab are available prior to infusion of axicabtagene ciloleucel. Monitor patients at least daily for 7 days at the certified healthcare facility following infusion for signs and symptoms of CRS. Monitor patients for signs or symptoms of CRS for 4 weeks after infusion. Counsel patients to seek immediate medical attention should signs or symptoms of CRS occur at any time. At the first sign of CRS, institute treatment with supportive care, tocilizumab or tocilizumab and corticosteroids as indicated.
Neurologic Toxicities
- Neurologic toxicities, that were fatal or life-threatening, occurred following treatment with axicabtagene ciloleucel. Neurologic toxicities occurred in 87% of patients. Ninety-eight percent of all neurologic toxicities occurred within the first 8 weeks of axicabtagene ciloleucel infusion, with a median time to onset of 4 days (range: 1 to 43 days). The median duration of neurologic toxicities was 17 days. Grade 3 or higher neurologic toxicities occurred in 31% of patients.
- The most common neurologic toxicities included encephalopathy (57%), headache (44%), tremor (31%), dizziness (21%), aphasia (18%), delirium (17%), insomnia (9%) and anxiety (9%). Prolonged encephalopathy lasting up to 173 days was noted. Serious events including leukoencephalopathy and seizures occurred with axicabtagene ciloleucel. Fatal and serious cases of cerebral edema have occurred in patients treated with axicabtagene ciloleucel.
- Monitor patients at least daily for 7 days at the certified healthcare facility following infusion for signs and symptoms of neurologic toxicities. Monitor patients for signs or symptoms of neurologic toxicities for 4 weeks after infusion and treat promptly.
Axicabtagene Ciloleucel REMS
- Because of the risk of CRS and neurologic toxicities, axicabtagene ciloleucel is available only through a restricted program under a Risk Evaluation and Mitigation Strategy (REMS) called the axicabtagene ciloleucel REMS. The required components of the axicabtagene ciloleucel REMS are:
- Healthcare facilities that dispense and administer axicabtagene ciloleucel must be enrolled and comply with the REMS requirements. Certified healthcare facilities must have on-site, immediate access to tocilizumab, and ensure that a minimum of two doses of tocilizumab are available for each patient for infusion within 2 hours after axicabtagene ciloleucel infusion, if needed for treatment of CRS.
- Certified healthcare facilities must ensure that healthcare providers who prescribe, dispense or administer axicabtagene ciloleucel are trained about the management of CRS and neurologic toxicities.
- Further information is available at www.YescartaREMS.com or 1-844-454-KITE (5483).
Hypersensitivity Reactions
- Allergic reactions may occur with the infusion of axicabtagene ciloleucel. Serious hypersensitivity reactions including anaphylaxis, may be due to dimethyl sulfoxide (DMSO) or residual gentamicin in axicabtagene ciloleucel.
Serious Infections
- Severe or life-threatening infections occurred in patients after axicabtagene ciloleucel infusion. In Study 1, infections (all grades) occurred in 38% of patients. Grade 3 or higher infections occurred in 23% of patients. Grade 3 or higher infections with an unspecified pathogen occurred in 16% of patients, bacterial infections in 9%, and viral infections in 4%. Axicabtagene ciloleucel should not be administered to patients with clinically significant active systemic infections. Monitor patients for signs and symptoms of infection before and after axicabtagene ciloleucel infusion and treat appropriately. Administer prophylactic anti-microbials according to local guidelines.
- Febrile neutropenia was observed in 36% of patients after axicabtagene ciloleucel infusion and may be concurrent with CRS. In the event of febrile neutropenia, evaluate for infection and manage with broad spectrum antibiotics, fluids and other supportive care as medically indicated.
Viral Reactivation
- Hepatitis B virus (HBV) reactivation, in some cases resulting in fulminant hepatitis, hepatic failure and death, can occur in patients treated with drugs directed against B cells. Perform screening for HBV, HCV, and HIV in accordance with clinical guidelines before collection of cells for manufacturing.
Prolonged Cytopenias
- Patients may exhibit cytopenias for several weeks following lymphodepleting chemotherapy and axicabtagene ciloleucel infusion. In Study 1, Grade 3 or higher cytopenias not resolved by Day 30 following axicabtagene ciloleucel infusion occurred in 28% of patients and included thrombocytopenia (18%), neutropenia (15%), and anemia (3%). Monitor blood counts after axicabtagene ciloleucel infusion.
Hypogammaglobulinemia
- B-cell aplasia and hypogammaglobulinemia can occur in patients receiving treatment with axicabtagene ciloleucel. In Study 1, hypogammaglobulinemia occurred in 15% of patients. Monitor immunoglobulin levels after treatment with axicabtagene ciloleucel and manage using infection precautions, antibiotic prophylaxis and immunoglobulin replacement.
- The safety of immunization with live viral vaccines during or following axicabtagene ciloleucel treatment has not been studied. Vaccination with live virus vaccines is not recommended for at least 6 weeks prior to the start of lymphodepleting chemotherapy, during axicabtagene ciloleucel treatment, and until immune recovery following treatment with axicabtagene ciloleucel.
Secondary Malignancies
- Patients treated with axicabtagene ciloleucel may develop secondary malignancies. Monitor life-long for secondary malignancies. In the event that a secondary malignancy occurs, contact Kite at 1-844-454-KITE (5483) to obtain instructions on patient samples to collect for testing.
Effects on Ability to Drive and Use Machines
- Due to the potential for neurologic events, including altered mental status or seizures, patients receiving axicabtagene ciloleucel are at risk for altered or decreased consciousness or coordination in the 8 weeks following axicabtagene ciloleucel infusion. Advise patients to refrain from driving and engaging in hazardous occupations or activities, such as operating heavy or potentially dangerous machinery, during this initial period.
Adverse Reactions
Clinical Trials Experience
- Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
- The safety data described in this section reflect exposure to axicabtagene ciloleucel in the clinical trial (Study 1) in which 108 patients with relapsed/refractory B-cell NHL received CAR-positive T cells based on a recommended dose which was weight-based. Patients with a history of CNS disorders (such as seizures or cerebrovascular ischemia) or autoimmune disease requiring systemic immunosuppression were ineligible. The median duration of follow up was 8.7 months. The median age of the study population was 58 years (range: 23 to 76 years); 68% were men. The baseline ECOG performance status was 43% with ECOG 0, and 57% with ECOG 1.
- The most common adverse reactions (incidence ≥ 20%) include CRS, fever, hypotension, encephalopathy, tachycardia, fatigue, headache, decreased appetite, chills, diarrhea, febrile neutropenia, infections-pathogen unspecified, nausea, hypoxia, tremor, cough, vomiting, dizziness, constipation, and cardiac arrhythmias. Serious adverse reactions occurred in 52% of patients. The most common serious adverse reactions (> 2%) include encephalopathy, fever, lung infection, febrile neutropenia, cardiac arrhythmia, cardiac failure, urinary tract infection, renal insufficiency, aphasia, cardiac arrest, Clostridium difficile infection, delirium, hypotension, and hypoxia.
- The most common (≥ 10%) Grade 3 or higher reactions include febrile neutropenia, fever, CRS, encephalopathy, infections-pathogen unspecified, hypotension, hypoxia, and lung infections.
- Forty-five percent (49/108) of patients received tocilizumab after infusion of axicabtagene ciloleucel.
- Table 3 summarizes the adverse reactions that occurred in at least 10% of patients treated with axicabtagene ciloleucel and Table 4 describes the laboratory abnormalities of Grade 3 or 4 that occurred in at least 10% of patients.
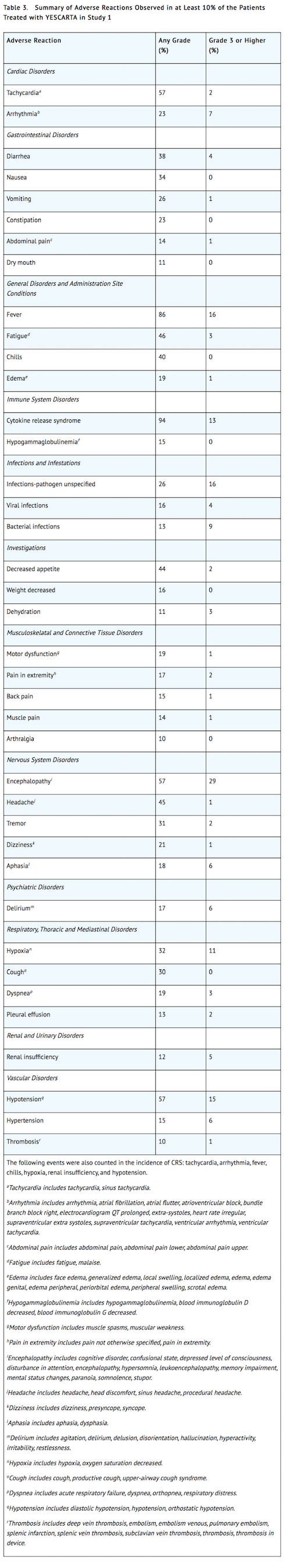
- Other clinically important adverse reactions that occurred in less than 10% of patients treated with axicabtagene ciloleucel include the following:
- Blood and lymphatic system disorders: Coagulopathy (2%).
- Cardiac disorders: Cardiac failure (6%) and cardiac arrest (4%).
- Immune system disorders: Hemophagocytic lymphohistiocytosis/macrophage activation syndrome (HLH/MAS) (1%), hypersensitivity (1%).
- Infections and infestations disorders: Fungal infections (5%).
- Nervous system disorders: Ataxia (6%), seizure (4%), dyscalculia (2%), and myoclonus (2%).
- Respiratory, thoracic and mediastinal disorders: Pulmonary edema (9%).
- Skin and subcutaneous tissue disorders: Rash (9%).
- Vascular disorders: Capillary leak syndrome (3%).
Laboratory Abnormalities

Immunogenicity
- Axicabtagene ciloleucel has the potential to induce anti-product antibodies. The immunogenicity of axicabtagene ciloleucel has been evaluated using an enzyme-linked immunosorbent assay (ELISA) for the detection of binding antibodies against FMC63, the originating antibody of the anti-CD19 CAR. Three patients tested positive for pre-dose anti-FMC63 antibodies at baseline and Months 1, 3, or 6 in Study 1. There is no evidence that the kinetics of initial expansion and persistence of axicabtagene ciloleucel, or the safety or effectiveness of axicabtagene ciloleucel, was altered in these patients.
Postmarketing Experience
There is limited information regarding Axicabtagene ciloleucel Postmarketing Experience in the drug label.
Drug Interactions
There is limited information regarding Axicabtagene ciloleucel Drug Interactions in the drug label.
Use in Specific Populations
Pregnancy
- There are no available data with axicabtagene ciloleucel use in pregnant women. No animal reproductive and developmental toxicity studies have been conducted with axicabtagene ciloleucel to assess whether it can cause fetal harm when administered to a pregnant woman. It is not known if axicabtagene ciloleucel has the potential to be transferred to the fetus. Based on the mechanism of action, if the transduced cells cross the placenta, they may cause fetal toxicity, including B-cell lymphocytopenia. Therefore, axicabtagene ciloleucel is not recommended for women who are pregnant, and pregnancy after axicabtagene ciloleucel infusion should be discussed with the treating physician.
- In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2%-4% and 15%-20%, respectively.
Pregnancy Category (AUS):
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Axicabtagene ciloleucel in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Axicabtagene ciloleucel during labor and delivery.
Nursing Mothers
There is no information regarding the presence of axicabtagene ciloleucel in human milk, the effect on the breastfed infant, and the effects on milk production. The developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for axicabtagene ciloleucel and any potential adverse effects on the breastfed infant from axicabtagene ciloleucel or from the underlying maternal condition.
Pediatric Use
The safety and efficacy of axicabtagene ciloleucel have not been established in pediatric patients.
Geriatic Use
Clinical trials of axicabtagene ciloleucel did not include sufficient numbers of patients aged 65 years and older to determine whether they respond differently or have different safety outcomes as compared to younger patients.
Gender
There is no FDA guidance on the use of Axicabtagene ciloleucel with respect to specific gender populations.
Race
There is no FDA guidance on the use of Axicabtagene ciloleucel with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Axicabtagene ciloleucel in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Axicabtagene ciloleucel in patients with hepatic impairment.
Females of Reproductive Potential and Males
Pregnancy Testing
- Pregnancy status of females with reproductive potential should be verified. Sexually-active females of reproductive potential should have a pregnancy test prior to starting treatment with axicabtagene ciloleucel.
Contraception
- See the prescribing information for fludarabine and cyclophosphamide for information on the need for effective contraception in patients who receive the lymphodepleting chemotherapy.
- There are insufficient exposure data to provide a recommendation concerning duration of contraception following treatment with axicabtagene ciloleucel.
Infertility
- There are no data on the effect of axicabtagene ciloleucel on fertility.
Immunocompromised Patients
There is no FDA guidance one the use of Axicabtagene ciloleucel in patients who are immunocompromised.
Administration and Monitoring
Administration
Preparing Patient for axicabtagene ciloleucel Infusion
- Confirm availability of axicabtagene ciloleucel prior to starting the lymphodepleting regimen.
Pre-treatment
- Administer a lymphodepleting chemotherapy regimen of cyclophosphamide 500 mg/m2 intravenously and fludarabine 30 mg/m2 intravenously on the fifth, fourth, and third day before infusion of axicabtagene ciloleucel.
Premedication
- Administer acetaminophen 650 mg PO and diphenhydramine 12.5 mg intravenously or PO approximately 1 hour before axicabtagene ciloleucel infusion.
- Avoid prophylactic use of systemic corticosteroids, as it may interfere with the activity of axicabtagene ciloleucel.
Preparation of Axicabtagene Ciloleucel for Infusion
- Coordinate the timing of axicabtagene ciloleucel thaw and infusion. Confirm the infusion time in advance, and adjust the start time of axicabtagene ciloleucel thaw such that it will be available for infusion when the patient is ready.
- Confirm patient identity: Prior to axicabtagene ciloleucel preparation, match the patient’s identity with the patient identifiers on the axicabtagene ciloleucel cassette.
- Do not remove the axicabtagene ciloleucel product bag from the cassette if the information on the patient-specific label does not match the intended patient.
- Once patient identification is confirmed, remove the axicabtagene ciloleucel product bag from the cassette and check that the patient information on the cassette label matches the bag label.
- Inspect the product bag for any breaches of container integrity such as breaks or cracks before thawing. If the bag is compromised, follow the local guidelines (or call Kite at 1-844-454-KITE).
- Place the infusion bag inside a second sterile bag per local guidelines.
- Thaw axicabtagene ciloleucel at approximately 37°C using either a water bath or dry thaw method until there is no visible ice in the infusion bag. Gently mix the contents of the bag to disperse clumps of cellular material. If visible cell clumps remain continue to gently mix the contents of the bag. Small clumps of cellular material should disperse with gentle manual mixing. Do not wash, spin down, and/or re-suspend axicabtagene ciloleucel in new media prior to infusion.
- Once thawed, axicabtagene ciloleucel may be stored at room temperature (20°C to 25°C) for up to 3 hours.
Administration
- Axicabtagene ciloleucel is for autologous use only. The patient’s identity must match the patient identifiers on the axicabtagene ciloleucel cassette and infusion bag. Do not infuse axicabtagene ciloleucel if the information on the patient-specific label does not match the intended patient.
- For autologous use only.
- Ensure that tocilizumab and emergency equipment are available prior to infusion and during the recovery period.
- Do NOT use a leukodepleting filter.
- Central venous access is recommended for the infusion of axicabtagene ciloleucel.
- Confirm the patient’s identity matches the patient identifiers on the axicabtagene ciloleucel product bag.
- Prime the tubing with normal saline prior to infusion.
- Infuse the entire contents of the axicabtagene ciloleucel bag within 30 minutes by either gravity or a peristaltic pump. Axicabtagene ciloleucel is stable at room temperature for up to 3 hours after thaw.
- Gently agitate the product bag during axicabtagene ciloleucel infusion to prevent cell clumping.
- After the entire content of the product bag is infused, rinse the tubing with normal saline at the same infusion rate to ensure all product is delivered.
- Axicabtagene ciloleucel contains human blood cells that are genetically modified with replication incompetent retroviral vector. Follow universal precautions and local biosafety guidelines for handling and disposal to avoid potential transmission of infectious diseases.
Monitoring
- Administer axicabtagene ciloleucel at a certified healthcare facility.
- Monitor patients at least daily for 7 days at the certified healthcare facility following infusion for signs and symptoms of CRS and neurologic toxicities.
- Instruct patients to remain within proximity of the certified healthcare facility for at least 4 weeks following infusion.
Management of Severe Adverse Reactions
Cytokine Release Syndrome
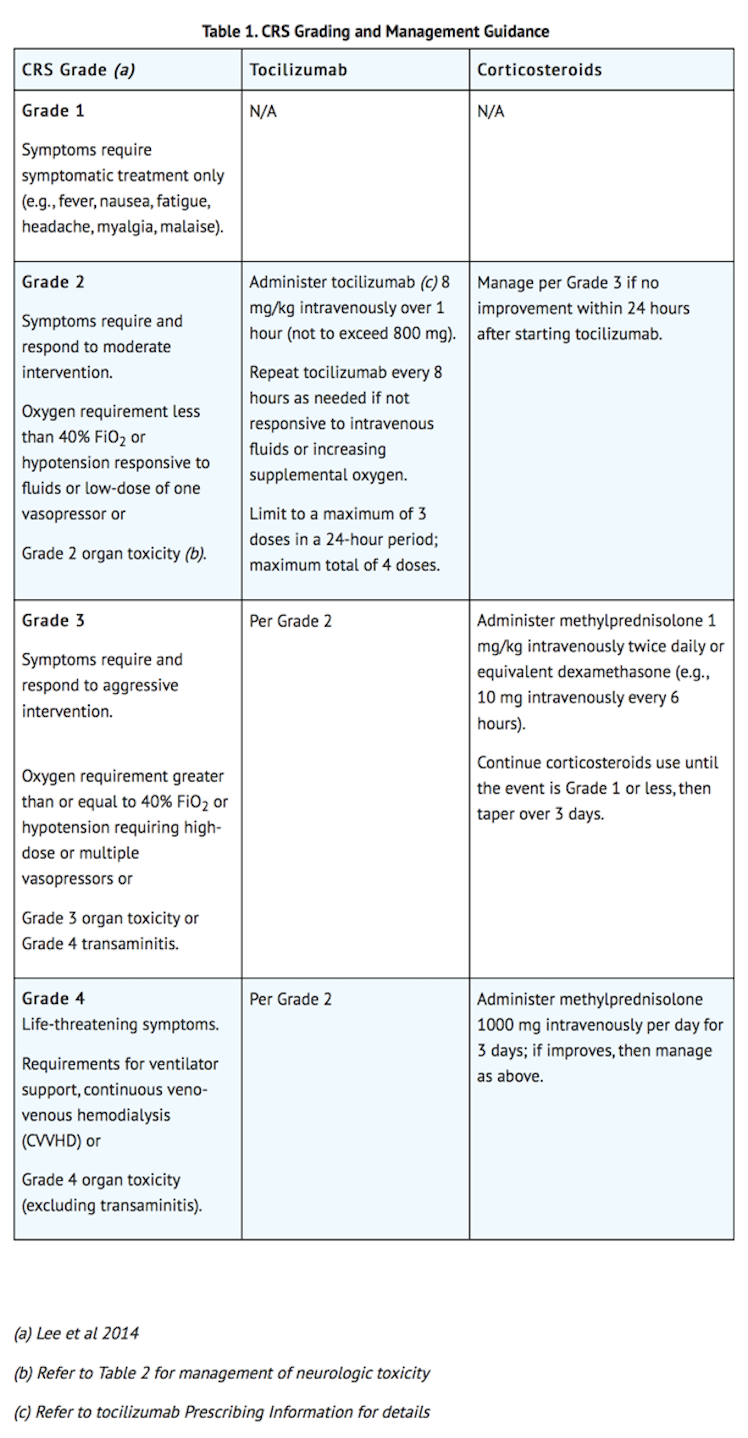
- Identify CRS based on clinical presentation. Evaluate for and treat other causes of fever, hypoxia, and hypotension. If CRS is suspected, manage according to the recommendations in Table 1. Patients who experience Grade 2 or higher CRS (e.g., hypotension, not responsive to fluids, or hypoxia requiring supplemental oxygenation) should be monitored with continuous cardiac telemetry and pulse oximetry. For patients experiencing severe CRS, consider performing an echocardiogram to assess cardiac function. For severe or life-threatening CRS, consider intensive care supportive therapy.
Neurologic Toxicity
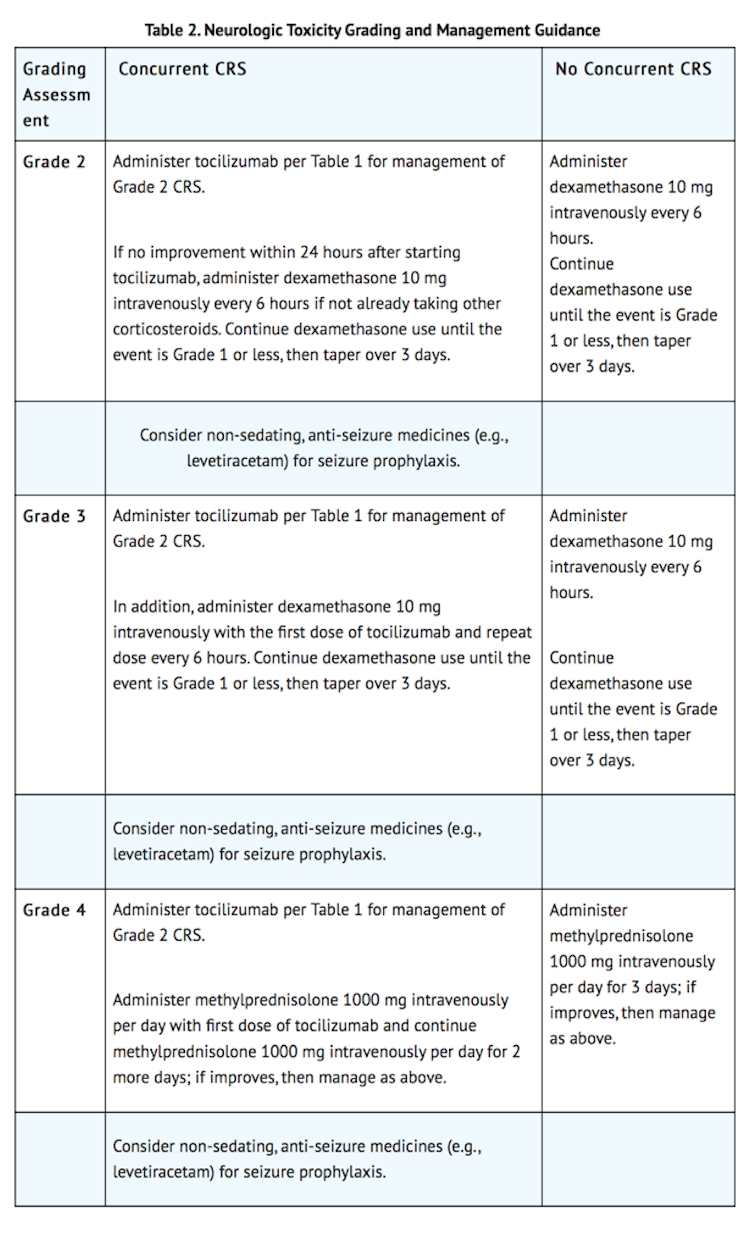
- Monitor patients for signs and symptoms of neurologic toxicities (Table 2). Rule out other causes of neurologic symptoms. Patients who experience Grade 2 or higher neurologic toxicities should be monitored with continuous cardiac telemetry and pulse oximetry. Provide intensive care supportive therapy for severe or life threatening neurologic toxicities. Consider non-sedating, anti-seizure medicines (e.g., levetiracetam) for seizure prophylaxis for any Grade 2 or higher neurologic toxicities.
IV Compatibility
There is limited information regarding the compatibility of Axicabtagene ciloleucel and IV administrations.
Overdosage
There is limited information regarding Axicabtagene ciloleucel overdosage. If you suspect drug poisoning or overdose, please contact the National Poison Help hotline (1-800-222-1222) immediately.
Pharmacology
Axicabtagene ciloleucel
| |
| Systematic (IUPAC) name | |
| ? | |
| Identifiers | |
| CAS number | ? |
| ATC code | None |
| PubChem | ? |
| DrugBank | |
| Chemical data | |
| Formula | ? |
| Mol. mass | ? |
| Pharmacokinetic data | |
| Bioavailability | ? |
| Metabolism | ? |
| Half life | ? |
| Excretion | ? |
| Therapeutic considerations | |
| Pregnancy cat. |
? |
| Legal status |
[[Prescription drug|Template:Unicode-only]](US) |
| Routes | Intravenous injection |
Mechanism of Action
- Axicabtagene ciloleucel, a CD19-directed genetically modified autologous T cell immunotherapy, binds to CD19-expressing cancer cells and normal B cells. Studies demonstrated that following anti-CD19 CAR T cell engagement with CD19-expressing target cells, the CD28 and CD3-zeta co-stimulatory domains activate downstream signaling cascades that lead to T cell activation, proliferation, acquisition of effector functions and secretion of inflammatory cytokines and chemokines. This sequence of events leads to killing of CD19-expressing cells.
Structure
There is limited information regarding Axicabtagene ciloleucel Structure in the drug label.
Pharmacodynamics
- After axicabtagene ciloleucel infusion, pharmacodynamic responses were evaluated over a 4-week interval by measuring transient elevation of cytokines, chemokines and other molecules in blood. Levels of cytokines and chemokines such as IL-6, IL-8, IL-10, IL-15, TNF-α, IFN-γ, and sIL2Rα were analyzed. Peak elevation was observed within the first 14 days after infusion, and levels generally returned to baseline within 28 days.
- Due to the on-target effect of axicabtagene ciloleucel, a period of B-cell aplasia is expected.
Pharmacokinetics
- Following infusion of axicabtagene ciloleucel, anti-CD19 CAR T cells exhibited an initial rapid expansion followed by a decline to near baseline levels by 3 months. Peak levels of anti-CD19 CAR T cells occurred within the first 7-14 days after axicabtagene ciloleucel infusion.
- Age (range: 23 – 76 years) and gender had no significant impact on AUC Day 0 - 28 and Cmax of axicabtagene ciloleucel.
- The number of anti-CD19 CAR T cells in blood was positively associated with objective response [complete remission (CR) or partial remission (PR)]. The median anti-CD19 CAR T cell Cmax levels in responders (n=73) were 205% higher compared to the corresponding level in nonresponders (n=23) (43.6 cells/μL vs 21.2 cells/μL). Median AUC Day 0 - 28 in responding patients (n=73) was 251% of the corresponding level in nonresponders (n=23) (557.1 days × cells/μL vs. 222.0 days × cells/μL).
- Some patients required tocilizumab and corticosteroids for management of CRS and neurologic toxicities. Patients treated with tocilizumab (n=44) had 262% and 232% higher anti-CD19 CAR T cells as measured by AUC Day 0 - 28 and Cmax respectively, as compared to patients who did not receive tocilizumab (n=57). Similarly, patients that received corticosteroids (n=26) had 217% and 155% higher AUC Day 0 - 28 and Cmax compared to patients who did not receive corticosteroids (n=75).
- Hepatic and renal impairment studies of axicabtagene ciloleucel were not conducted.
Nonclinical Toxicology
Carcinogenesis, Mutagenesis, Impairment of Fertility
- No carcinogenicity or genotoxicity studies have been conducted with axicabtagene ciloleucel. No studies have been conducted to evaluate the effects of axicabtagene ciloleucel on fertility.
Clinical Studies
Relapsed or Refractory Large B-Cell Lymphoma
- A single-arm, open-label, multicenter trial evaluated the efficacy of a single infusion of axicabtagene ciloleucel in adult patients with relapsed or refractory aggressive B-cell non-Hodgkin lymphoma. Eligible patients had refractory disease to the most recent therapy or relapse within 1 year after autologous hematopoietic stem cell transplantation (HSCT). The study excluded patients with prior allogeneic HSCT, any history of central nervous system lymphoma, ECOG performance status of 2 or greater, absolute lymphocyte count less than 100/µL, creatinine clearance less than 60 mL/min, hepatic transaminases more than 2.5 times the upper limit of normal, cardiac ejection fraction less than 50%, or active serious infection.
- Following lymphodepleting chemotherapy, axicabtagene ciloleucel was administered as a single intravenous infusion at a target dose of 2 × 106 CAR-positive viable T cells/kg (maximum permitted dose: 2 × 108 cells). The lymphodepleting regimen consisted of cyclophosphamide 500 mg/m2 intravenously and fludarabine 30 mg/m2 intravenously, both given on the fifth, fourth, and third day before axicabtagene ciloleucel. Bridging chemotherapy between leukapheresis and lymphodepleting chemotherapy was not permitted. All patients were hospitalized for axicabtagene ciloleucel infusion and for a minimum of 7 days afterward.
- Of 111 patients who underwent leukapheresis, 101 received axicabtagene ciloleucel. Of the patients treated, the median age was 58 years (range: 23 to 76), 67% were male, and 89% were white. Most (76%) had DLBCL, 16% had transformed follicular lymphoma, and 8% had primary mediastinal large B-cell lymphoma. The median number of prior therapies was 3 (range: 1 to 10), 77% of the patients had refractory disease to a second or greater line of therapy, and 21% had relapsed within 1 year of autologous HSCT.
- One out of 111 patients did not receive the product due to manufacturing failure. Nine other patients were not treated, primarily due to progressive disease or serious adverse reactions following leukapheresis. The median time from leukapheresis to product delivery was 17 days (range: 14 to 51 days), and the median time from leukapheresis to infusion was 24 days (range: 16 to 73 days). The median dose was 2.0 × 106 CAR-positive viable T cells/kg (range: 1.1 to 2.2 × 106 cells/kg).
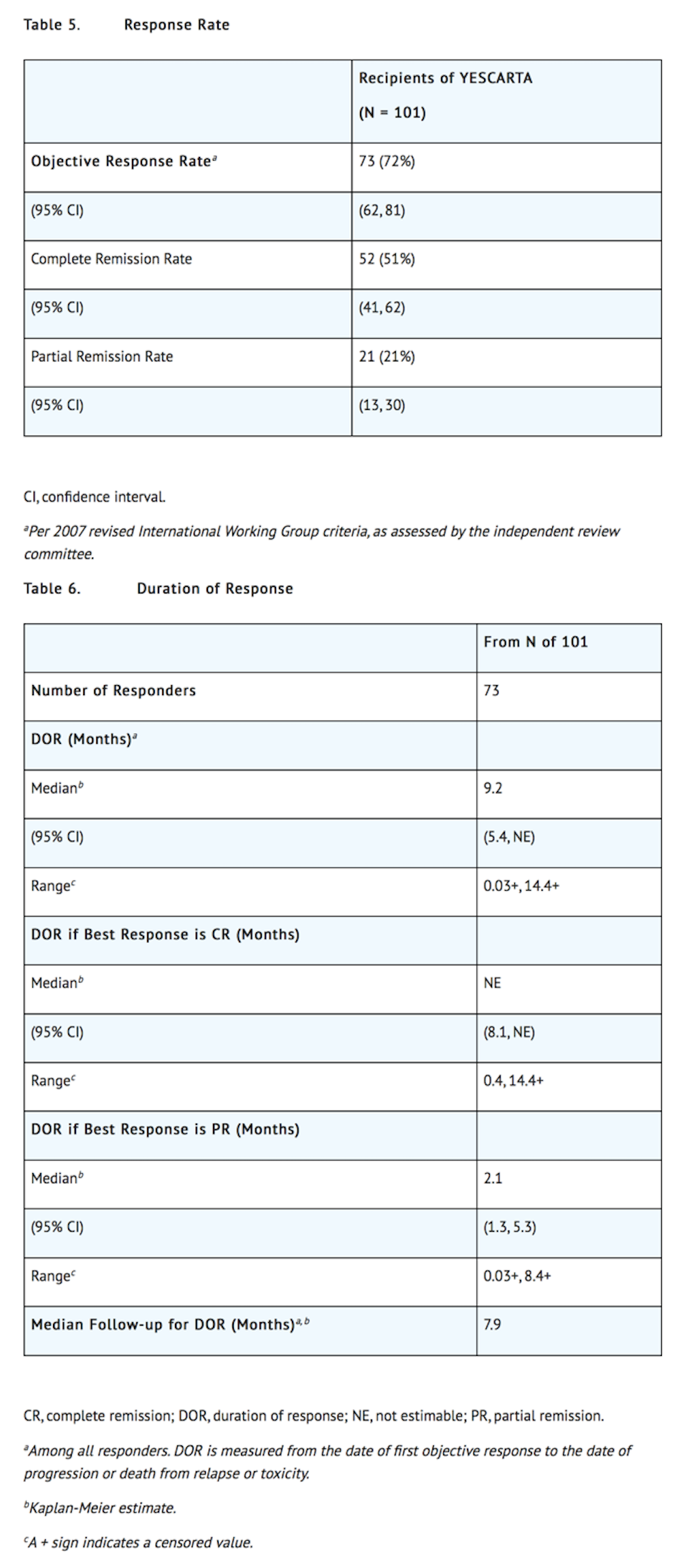
- Efficacy was established on the basis of complete remission (CR) rate and duration of response (DOR), as determined by an independent review committee (Table 5 and Table 6). The median time to response was 0.9 months (range: 0.8 to 6.2 months). Response durations were longer in patients who achieved CR, as compared to patients with a best response of partial remission (PR) (Table 6). Of the 52 patients who achieved CR, 14 initially had stable disease (7 patients) or PR (7 patients), with a median time to improvement of 2.1 months (range: 1.6 to 5.3 months).
How Supplied
- Axicabtagene ciloleucel is supplied in an infusion bag (NDC 71287-119-01) containing approximately 68 mL of frozen suspension of genetically modified autologous T cells in 5% DMSO and 2.5% albumin (human).
Storage
- Each axicabtagene ciloleucel infusion bag is individually packed in a metal cassette (NDC 71287-119-02). Axicabtagene ciloleucel is stored in the vapor phase of liquid nitrogen and supplied in a liquid nitrogen dry shipper.
- Match the identity of the patient with the patient identifiers on the cassette and infusion bag upon receipt.
- Store axicabtagene ciloleucel frozen in the vapor phase of liquid nitrogen (less than or equal to minus 150ºC).
- Thaw before using.
Images
Drug Images
{{#ask: Page Name::Axicabtagene ciloleucel |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel


{{#ask: Label Page::Axicabtagene ciloleucel |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
- Ensure that patients understand the risk of manufacturing failure (1% in clinical trial). In case of a manufacturing failure, a second manufacturing of axicabtagene ciloleucel may be attempted. In addition, while the patient awaits the product, additional chemotherapy (not the lymphodepletion) may be necessary and may increase the risk of adverse events during the pre-infusion period.
- Advise patients to seek immediate attention for any of the following:
- Cytokine Release Syndrome (CRS) - Signs or symptoms associated with CRS including fever, chills, fatigue, tachycardia, nausea, hypoxia, and hypotension.
- Neurologic Toxicities – Signs or symptoms associated with neurologic events including encephalopathy, seizures, changes in level of consciousness, speech disorders, tremors, and confusion.
- Serious Infections - Signs or symptoms associated with infection.
- Prolonged Cytopenia - Signs or symptoms associated with bone marrow suppression including neutropenia, anemia, thrombocytopenia, or febrile neutropenia.
- Advise patients for the need to:
- Refrain from driving or operating heavy or potentially dangerous machinery after axicabtagene ciloleucel infusion until at least 8 weeks after infusion.
- Have periodic monitoring of blood counts.
- Contact Kite at 1-844-454-KITE (5483) if they are diagnosed with a secondary malignancy.
Precautions with Alcohol
Alcohol-Axicabtagene ciloleucel interaction has not been established. Talk to your doctor regarding the effects of taking alcohol with this medication.
Brand Names
- Yescarta
Look-Alike Drug Names
There is limited information regarding Axicabtagene ciloleucel Look-Alike Drug Names in the drug label.
Drug Shortage
Price
References
The contents of this FDA label are provided by the National Library of Medicine.