Amoxicillin detailed information
 | |
 | |
| Clinical data | |
|---|---|
| Pregnancy category | |
| Routes of administration | Oral, intravenous |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | 95% oral |
| Metabolism | less than 30% biotransformed in liver |
| Elimination half-life | 61.3 minutes |
| Excretion | renal |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| E number | {{#property:P628}} |
| ECHA InfoCard | {{#property:P2566}}Lua error in Module:EditAtWikidata at line 36: attempt to index field 'wikibase' (a nil value). |
| Chemical and physical data | |
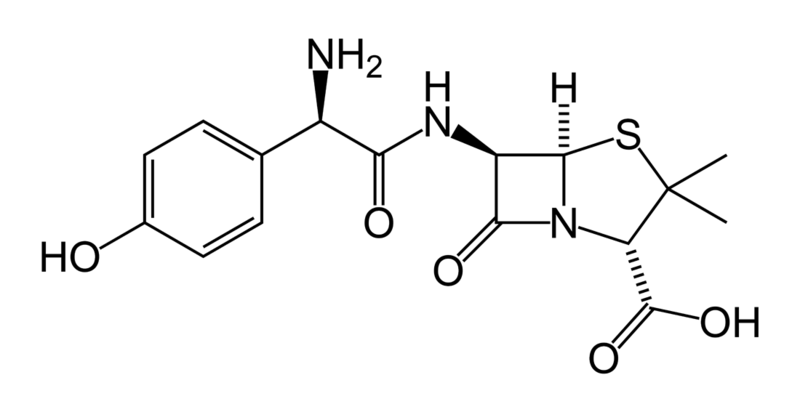
| Formula | C16H19N3O5S |
| Molar mass | 365.4 g/mol |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]
Overview
Amoxicillin (INN) or amoxycillin (former BAN) is a moderate-spectrum β-lactam antibiotic used to treat bacterial infections caused by susceptible microorganisms. It is usually the drug of choice within the class because it is better absorbed, following oral administration, than other beta-lactam antibiotics. Amoxicillin is susceptible to degradation by β-lactamase-producing bacteria, and so may be given with clavulanic acid to decrease its susceptibility (see below). It was developed by Beecham in 1972 and is currently marketed by GlaxoSmithKline (the inheritor company) under the original trade name Amoxil.
Mode of action
Amoxicillin acts by inhibiting the synthesis of bacterial cell walls. It inhibits cross-linkage between the linear peptidoglycan polymer chains that make up a major component of the cell wall of Gram-positive bacteria.
Microbiology
Amoxicillin is a moderate-spectrum antibiotic active against a wide range of Gram-positive, and a limited range of Gram-negative organisms. Some examples of susceptible and resistant organisms, from the Amoxil® Approved Product Information (GSK, 2003), are listed below.
Susceptible Gram-positive organisms
Streptococcus spp., penicillin-susceptible Streptococcus pneumoniae, non β-lactamase-producing Staphylococcus spp., and Enterococcus faecalis.
Susceptible Gram-negative organisms
Non-β-lactamase producing strains of the following bacteria: Haemophilus influenzae, Neisseria gonorrhoeae, Neisseria meningitidis, Escherichia coli, Proteus mirabilis and Salmonella spp.
Resistant organisms
Penicillinase-producing organisms, particularly penicillinase-producing Staphylococcus spp. Penicillinase-producing N. gonorrhoeae and H. influenzae are also resistant.
All strains of Pseudomonas spp., Klebsiella spp., Enterobacter spp., indole-positive Proteus spp., Serratia marcescens, and Citrobacter spp. are resistant.
The incidence of β-lactamase-producing resistant organisms, including E. coli, appears to be increasing.
Doubling the routinely given concentration (in pediatrics) of amoxicillin has been shown to eradicate intermediately resistant Streptococcus pneumoniae in selected infections.[1]
Formulations
Amoxicillin in trihydrate form is available as capsules, tablets, or syrup for oral use, and as the sodium salt for intravenous administration. It is one of the most common antibiotics issued to children. It has 3 ionizable groups.
Amoxicillin and clavulanic acid
Amoxicillin (in either trihydrate or sodium salt forms) may be combined with Clavulanic acid (as potassium clavulanate), a β-lactamase inhibitor, to increase the spectrum of action against Gram-negative organisms, and to overcome bacterial antibiotic resistance mediated through β-lactamase production. This formulation is referred to as Co-amoxiclav (British Approved Name), but commonly by proprietary names such as Amoksiklav (×2 or SOLVO), Augmentin, Clamoxyl, Augclav, and Augmexx.
Side effects
Side effects are as those for other beta-lactam antibiotics. Side effects include nausea, vomiting and easy fatigue.
The onset of an allergic reaction to Amoxicillin can be very sudden and intense - emergency medical attention must be sought as quickly as possible. The initial onset of such a reaction often starts with a change in mental state; skin rash with intense itching (often beginning in fingertips and around groin area and rapidly spreading) and sensations of fever, nausea and vomiting. Any other symptoms that seem even remotely suspicious must be taken very seriously.
Proprietary preparations
The patent for amoxicillin has expired. Thus amoxicillin is marketed under many trade names including: Actimoxi, Amoksibos, Amoxiclav Sandoz, Amoxil, Amoksiklav, Amoxibiotic, Amoxicilina, Apo-Amoxi, Bactox, Betalaktam, Cilamox, Curam, Dedoxil, Dispermox, Duomox, Isimoxin, Klavox, Lamoxy, Moxypen, Moxyvit, Novamoxin, Ospamox, Panklav, Pamoxicillin, Polymox, Samthongcillin, Senox, Sinacilin, Trimox, Tolodina, Wymox, Zerrsox and Zimox.
References
- ↑ 2003 Red Book: Report of the Committee on Infectious Diseases. Elk Grove Village, Illinois: American Academy of Pediatrics. 2003. ISBN 1-58110-095-7.
See also
Additional Resources
- GlaxoSmithKline (2006). "Amoxil - Prescribing information" (PDF). Unknown parameter
|month=ignored (help) - Neal, MJ (2002). Medical Pharmacology at a Glance (4 ed.). Oxford: Blackwell Science. ISBN 0-632-05244-9
- British National Formulary 45 March 2003
bg:Амоксицилин ca:Amoxicil·lina de:Amoxicillin gl:Amoxicilina ko:아목시실린 he:אמוקסיצילין hu:Amoxicillin nl:Amoxicilline sr:Амоксицилин su:Amoxicillin fi:Amoksisilliini th:อะม็อกซีซิลลิน
- Pages with script errors
- E number from Wikidata
- ECHA InfoCard ID from Wikidata
- Chemical articles with unknown parameter in Infobox drug
- Articles without EBI source
- Chemical pages without ChemSpiderID
- Articles without KEGG source
- Articles without InChI source
- Articles without UNII source
- Articles containing unverified chemical infoboxes
- Pages with citations using unsupported parameters
- Beta-lactam antibiotics