Adenoviridae
| Adenoviruses | ||||
|---|---|---|---|---|
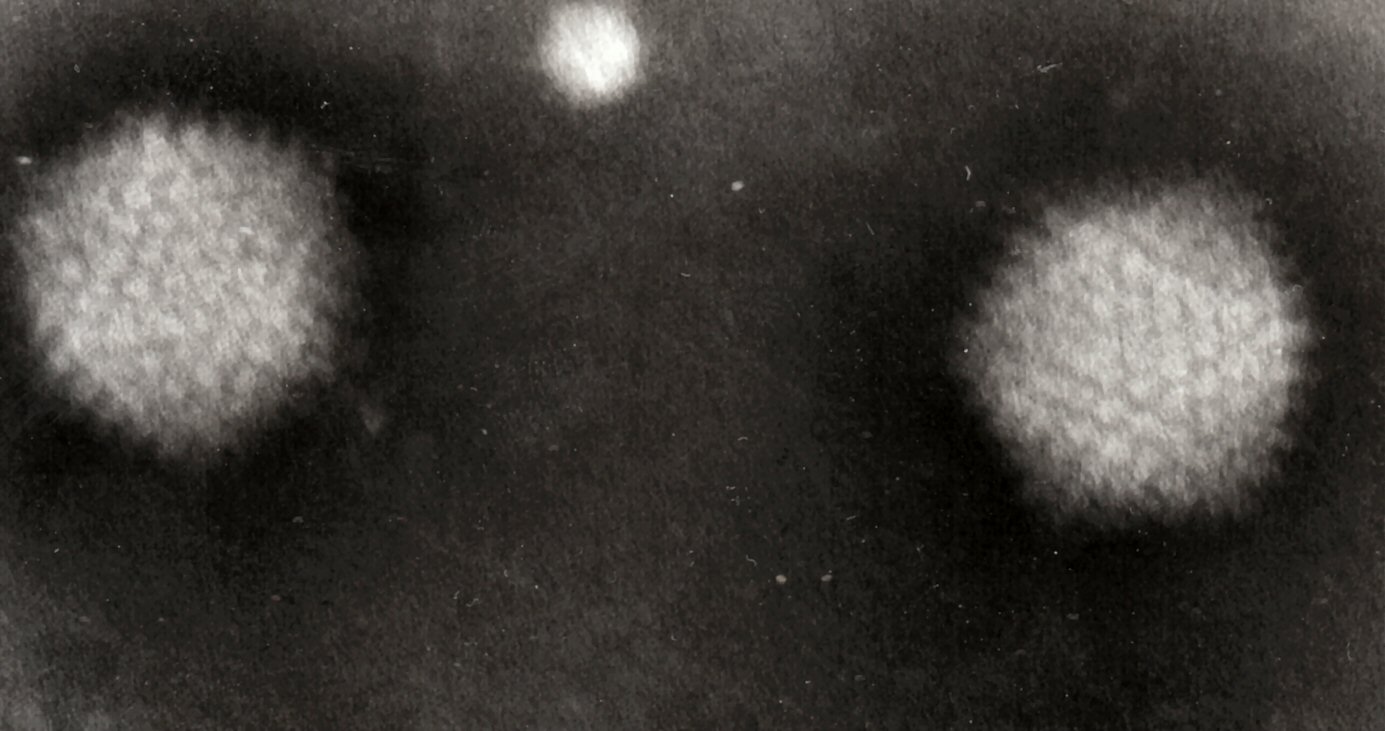 Transmission electron micrograph of two adenovirus particles
| ||||
| Virus classification | ||||
| ||||
| Genera | ||||
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1] Synonyms and keywords: Adenoviruses
Overview
Adenoviridae are medium-sized (90-100 nm), nonenveloped (naked) icosahedral viruses composed of a nucleocapsid and a double-stranded linear DNA genome. There are over 52 different serotypes in humans, which are responsible for 5–10% of upper respiratory infections in children, and many infections in adults as well.
Causes
Viruses of the family Adenoviridae infect various species of animals, including humans. Adenoviruses were first isolated in human adenoids (tonsils), from which the name is derived, and are classified as group I under the Baltimore classification scheme. Adenoviruses represent the largest nonenveloped viruses, because they are the maximum size able to be transported through the endosome (i.e. envelope fusion is not necessary). The virion also has a unique "spike" or fiber associated with each penton base of the capsid (see picture below) that aids in attachment to the host cell via the coxsackie-adenovirus receptor on the surface of the host cell. There are 51 immunologically distinct human adenovirus serotypes (6 species: Human adenovirus A through F) that can cause human infections ranging from respiratory disease (mainly species HAdV-B and C), and conjunctivitis (HAdV-B and D), to gastroenteritis (HAdV-F serotypes 40 and 41). Adenoviruses are unusually stable to chemical or physical agents and adverse pH conditions, allowing for prolonged survival outside of the body and water. Adenoviruses are primarily spread via respiratory droplets, however they can also be spread by fecal routes as well. Most infections with adenovirus result in infections of the upper respiratory tract. Adenovirus infections often show up as conjunctivitis, tonsilitis (which may look exactly like strep throat and cannot be distinguished from strep except by throat culture), an ear infection, or croup. Adenoviruses can also cause gastroenteritis (stomach flu). A combination of conjunctivitis and tonsilitis is particularly common with adenovirus infections. Some children (especially small ones) can develop adenovirus bronchiolitis or pneumonia, both of which can be severe. In babies, adenoviruses can also cause coughing fits that look almost exactly like whooping cough. Adenoviruses can also cause viral meningitis or encephalitis. Rarely, adenovirus can cause cystitis (inflammation of the urinary bladder—a form of urinary tract infection—with blood in the urine). Most people recover from adenovirus infections by themselves, but people with immunodeficiency sometimes die of adenovirus infections, and—rarely—even previously healthy people can die of these infections.[1] Adenoviruses are often transmitted by coughed-out droplets, but can also be transmitted by contact with an infected person, or by virus particles left on objects such as towels and faucet handles. Some people with adenovirus gastroenteritis may shed the virus in their stools for months after getting over the symptoms. The virus can be passed from one person to another through some sexual practices, and through water in swimming pools that do not have enough chlorine in them. As with many other illnesses, good handwashing is one way to lessen the spread of adenoviruses from one person to another. Heat and bleach will kill adenoviruses on objects.
Genera
This family contains the following genera:
- Genus Aviadenovirus; type species: Fowl adenovirus A
- Genus Atadenovirus; type species: Ovine adenovirus D
- Genus Mastadenovirus; type species: Human adenovirus C; others include AD-36
- Genus Siadenovirus; type species: Frog adenovirus
Adenoviruses in humans
Genome

The adenovirus genome is linear, non-segmented double stranded (ds) DNA which is around 30–38 Kbp. This allows the virus to theoretically carry 30 to 40 genes. Although this is significantly larger than other viruses in its Baltimore group it is still a very simple virus and is heavily reliant on the host cell for survival and replication. An interesting feature of this viral genome is that it has a terminal 55 kDa protein associated with each of the 5' ends of the linear dsDNA, these are used as primers in viral replication and ensure that the ends of the virus' linear genome are adequately replicated.
Replication
Adenoviruses possess a linear dsDNA genome and are able to replicate in the nucleus of mammalian cells using the host’s replication machinery.

Entry of adenoviruses into the host cell involves two sets of interactions between the virus and the host cell. Entry into the host cell is initiated by the knob domain of the fiber protein binding to the cell receptor. The two currently established receptors are: CD46 for the group B human adenovirus serotypes and the coxsackievirus adenovirus receptor (CAR) for all other serotypes. There are some reports suggesting MHC molecules and sialic acid residues functioning in this capacity as well. This is followed by a secondary interaction, where a specialized motif in the penton base protein interacts with an integrin molecule. It is the co-receptor interaction that stimulates internalization of the adenovirus. This co-receptor molecule is αv integrin. Binding to αv integrin results in endocytosis of the virus particle via clathrin-coated pits. Attachment to αv integrin stimulates cell signaling and thus induces actin polymerization resulting in entry of the virion into the host cell within an endosome.[2]
Once the virus has successfully gained entry into the host cell the endosome acidifies, which alters virus topology by causing capsid components to disassociate. These changes as well as the toxic nature of the pentons results in the release of the virion into the cytoplasm. With the help of cellular microtubules the virus is transported to the nuclear pore complex whereby the adenovirus particle disassembles. Viral DNA is subsequently released which can enter the nucleus via the nuclear pore.[3] After this the DNA associates with histone molecules. Thus viral gene expression can occur and new virus particles can be generated.
The adenovirus life cycle is separated, by the DNA replication process, into two phases: an early and a late phase. In both phases a primary transcript is generated which is alternatively spliced to generate monocistronic mRNAs compatible with the host’s ribosome, allowing for the products to be translated. The early genes are responsible for expressing mainly non-structural, regulatory proteins. The goal of these proteins is threefold: to alter the expression of host proteins that are necessary for DNA synthesis; to activate other virus genes (such as the virus-encoded DNA polymerase); and to avoid premature death of the infected cell by the host-immune defenses (blockage of apoptosis, blockage of interferon activity, and blockage of MHC class I translocation and expression). Some adenoviruses under specialized conditions can transform cells using their early gene products. E1a (binds Retinoblastoma tumor suppressor protein) has been found to immortalize primary cells in vitro allowing E1b (binds p53 tumor suppressor) to assist and stably transform the cells. Nevertheless, they are reliant upon each other to successfully transform the host cell and form tumors. DNA replication separates the early and late phases. Once the early genes have liberated adequate virus proteins, replication machinery and replication substrates, replication of the adenovirus genome can occur. A terminal protein that is covalently bound to the 5’ end of the adenovirus genome acts as a primer for replication. The viral DNA polymerase then uses a strand displacement mechanism, as opposed to the conventional Okazaki fragments used in mammalian DNA replication, to replicate the genome. The late phase of the adenovirus life cycle is focused on producing sufficient quantities of structural protein to pack all the genetic material produced by DNA replication. Once the viral components have successfully been replicated the virus is assembled into its protein shells and released from the cell as a result of virally induced cell lysis.
Differential diagnosis
Adenoviridae infection must be differentiated from other causes of viral, bacterial, and parasitic gastroentritis.
| Organism | Age predilection | Travel History | Incubation Size (cell) | Incubation Time | History and Symptoms | Diarrhea type8 | Food source | Specific consideration | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Fever | N/V | Cramping Abd Pain | Small Bowel | Large Bowel | Inflammatory | Non-inflammatory | |||||||||
| Viral | Rotavirus | <2 y | - | <102 | <48 h | + | + | - | + | + | - | Mostly in day cares, most common in winter. | |||
| Norovirus | Any age | - | 10 -103 | 24-48 h | + | + | + | + | + | - | Most common cause of gastroenteritis, abdominal tenderness, | ||||
| Adenovirus | <2 y | - | 105 -106 | 8-10 d | + | + | + | + | + | - | No seasonality | ||||
| Astrovirus | <5 y | - | 72-96 h | + | + | + | + | + | Seafood | Mostly during winter | |||||
| Bacterial | Escherichia coli | ETEC | Any age | + | 108 -1010 | 24 h | - | + | + | + | + | - | Causes travelers diarrhea, contains heat-labile toxins (LT) and heat-stable toxins (ST) | ||
| EPEC | <1 y | - | 10† | 6-12 h | - | + | + | + | + | Raw beef and chicken | - | ||||
| EIEC | Any ages | - | 10† | 24 h | + | + | + | + | + | Hamburger meat and unpasteurized milk | Similar to shigellosis, can cause bloody diarrhea | ||||
| EHEC | Any ages | - | 10 | 3-4 d | - | + | + | + | + | Undercooked or raw hamburger (ground beef) | Known as E. coli O157:H7, can cause HUS/TTP. | ||||
| EAEC | Any ages | + | 1010 | 8-18 h | - | - | + | + | + | - | May cause prolonged or persistent diarrhea in children | ||||
| Salmonella sp. | Any ages | + | 1 | 6 to 72 h | + | + | + | + | + | Meats, poultry, eggs, milk and dairy products, fish, shrimp, spices, yeast, coconut, sauces, freshly prepared salad. | Can cause salmonellosis or typhoid fever. | ||||
| Shigella sp. | Any ages | - | 10 - 200 | 8-48 h | + | + | + | + | + | Raw foods, for example, lettuce, salads (potato, tuna, shrimp, macaroni, and chicken) | Some strains produce enterotoxin and Shiga toxin similar to those produced by E. coli O157:H7 | ||||
| Campylobacter sp. | <5 y, 15-29 y | - | 104 | 2-5 d | + | + | + | + | + | Undercooked poultry products, unpasteurized milk and cheeses made from unpasteurized milk, vegetables, seafood and contaminated water. | May cause bacteremia, Guillain-Barré syndrome (GBS), hemolytic uremic syndrome (HUS) and recurrent colitis | ||||
| Yersinia enterocolitica | <10 y | - | 104 -106 | 1-11 d | + | + | + | + | + | Meats (pork, beef, lamb, etc.), oysters, fish, crabs, and raw milk. | May cause reactive arthritis; glomerulonephritis; endocarditis; erythema nodosum.
can mimic appendicitis and mesenteric lymphadenitis. | ||||
| Clostridium perfringens | Any ages | > 106 | 16 h | - | - | + | + | + | Meats (especially beef and poultry), meat-containing products (e.g., gravies and stews), and Mexican foods. | Can survive high heat, | |||||
| Vibrio cholerae | Any ages | - | 106-1010 | 24-48 h | - | + | + | + | + | Seafoods, including molluscan shellfish (oysters, mussels, and clams), crab, lobster, shrimp, squid, and finfish. | Hypotension, tachycardia, decreased skin turgor. Rice-water stools | ||||
| Parasites | Protozoa | Giardia lamblia | 2-5 y | + | 1 cyst | 1-2 we | - | - | + | + | + | Contaminated water | May cause malabsorption syndrome and severe weight loss | ||
| Entamoeba histolytica | 4-11 y | + | <10 cysts | 2-4 we | - | + | + | + | + | Contaminated water and raw foods | May cause intestinal amebiasis and amebic liver abscess | ||||
| Cryptosporidium parvum | Any ages | - | 10-100 oocysts | 7-10 d | + | + | + | + | + | Juices and milk | May cause copious diarrhea and dehydration in patients with AIDS especially with 180 > CD4 | ||||
| Cyclospora cayetanensis | Any ages | + | 10-100 oocysts | 7-10 d | - | + | + | + | + | Fresh produce, such as raspberries, basil, and several varieties of lettuce. | More common in rainy areas | ||||
| Helminths | Trichinella spp | Any ages | - | Two viable larvae (male and female) | 1-4 we | - | + | + | + | + | Undercooked meats | More common in hunters or people who eat traditionally uncooked meats | |||
| Taenia spp | Any ages | - | 1 larva or egg | 2-4 m | - | + | + | + | + | Undercooked beef and pork | Neurocysticercosis: Cysts located in the brain may be asymptomatic or seizures, increased intracranial pressure, headache. | ||||
| Diphyllobothrium latum | Any ages | - | 1 larva | 15 d | - | - | - | + | + | Raw or undercooked fish. | May cause vitamin B12 deficiency | ||||
8Small bowel diarrhea: watery, voluminous with less than 5 WBC/high power field
Large bowel diarrhea: Mucousy and/or bloody with less volume and more than 10 WBC/high power field
† It could be as high as 1000 based on patient's immunity system.
The table below summarizes the findings that differentiate inflammatory causes of chronic diarrhea[4][5][6][7][7]
| Cause | History | Laboratory findings | Diagnosis | Treatment |
|---|---|---|---|---|
| Diverticulitis |
|
|
Abdominal CT scan with oral and intravenous (IV) contrast | bowel rest, IV fluid resuscitation, and broad-spectrum antimicrobial therapy which covers anaerobic bacteria and gram-negative rods |
| Ulcerative colitis |
|
|
Endoscopy | Induction of remission with mesalamine and corticosteroids followed by the administration of sulfasalazine and 6-Mercaptopurine depending on the severity of the disease. |
| Entamoeba histolytica |
|
cysts shed with the stool | detects ameba DNA in feces | Amebic dysentery
Luminal amebicides for E. histolytica in the colon:
For amebic liver abscess:
|
Adenoviruses in animals
Two types of canine adenoviruses are well known, type 1 and 2. Type 1 causes infectious canine hepatitis, a potentially fatal disease involving vasculitis and hepatitis. Type 1 infection also can cause respiratory and eye infections. Canine adenovirus 2 (CAdV-2) is one of the potential causes of kennel cough. Core vaccines for dogs include attenuated live CAdV-2, which produces immunity to CAdV-1 and CAdV-2. CAdV-1 was initially used in a vaccine for dogs, but corneal edema was a common complication.[8]
Adenoviruses are also known to cause respiratory infections in horses, cattle, pigs, sheep, and goats. Equine adenovirus 1 can also cause fatal disease in immunocompromised Arabian foals, involving pneumonia and destruction of pancreatic and salivary gland tissue.[8]
Treatment and prevention
As with almost all viruses, there are no antibiotics that help with an adenoviral infection, so treatment is largely directed at the symptoms (such as acetaminophen for fever). A doctor may give antibiotic eyedrops for conjunctivitis, since it takes a while to test to see if the eye infection is bacterial or viral and to help prevent secondary bacterial infections. In the past, US military recruits were vaccinated against two serotypes of adenotypes, with a corresponding decrease in illnesses caused by those serotypes. The vaccine is no longer manufactured, and there are currently no vaccines available to protect against the adenovirus. Good hygiene, including handwashing, is still the best way to avoid picking up the adenovirus from an infected person.
Treatment Regimen
- Adenovirus[9]
- 1. In severe cases of pneumonia or post hematopoietic stem cell transplantation
- Preferred regimen (1): Cidofovir 5 mg/kg/week IV for 2 weeks, then every 2 weeks AND Probenecid 1.25 g/M2 PO given 3 hours before Cidofovir and 3 & 9 hours after each infusion
- Preferred regimen (2): Cidofovir 1 mg/kg IV 3 times per week
- Note: Ganciclovir, Foscarnet and Ribavirin are not recommended for use on adenovirus infection.[10]
- 2. For hemorrhagic cystitis
- 3. Pink eye (viral conjunctivitis)
- Preferred regimen: No specific treatment available. If symptomatic, cold artificial tears may help.
- 4.Bronchitis
- Preferred regimen: No specific therapy recommended, treatment is symptomatic.
See also
Template:Baltimore classification Template:Viral diseases
Sources
Centers for Disease Control and Prevention--National Center for Diseases--Division of Viral and Rickettsial Diseases, Respiratory and Enteric Viruses Branch
External links
- MicrobiologyBytes: Adenoviruses
- Adenoviruses General Concepts
- DNA virus replication strategies
- Sequenced adenoviruses
References
- ↑ Amy Burkholder (2007-12-19). "A killer cold? Even the healthy may be vulnerable". CNN. Retrieved 2007-12-19. Check date values in:
|date=(help) - ↑ Wu and Nemerow (2004). "Virus yoga: the role of flexibility in virus host cell recognition". Trends Microbiol. 12: 162–168. doi:10.1016/j.tim.2004.02.005. PMID 15051066.
- ↑ Meier and Greber (2004). "Adenovirus endocytosis". J Gene Med. 6: S152–S163. doi:10.1002/jgm.553. PMID 14978758.
- ↑ Konvolinka CW (1994). "Acute diverticulitis under age forty". Am J Surg. 167 (6): 562–5. PMID 8209928.
- ↑ Silverberg MS, Satsangi J, Ahmad T, Arnott ID, Bernstein CN, Brant SR; et al. (2005). "Toward an integrated clinical, molecular and serological classification of inflammatory bowel disease: report of a Working Party of the 2005 Montreal World Congress of Gastroenterology". Can J Gastroenterol. 19 Suppl A: 5A–36A. PMID 16151544.
- ↑ Satsangi J, Silverberg MS, Vermeire S, Colombel JF (2006). "The Montreal classification of inflammatory bowel disease: controversies, consensus, and implications". Gut. 55 (6): 749–53. doi:10.1136/gut.2005.082909. PMC 1856208. PMID 16698746.
- ↑ 7.0 7.1 Haque R, Huston CD, Hughes M, Houpt E, Petri WA (2003). "Amebiasis". N Engl J Med. 348 (16): 1565–73. doi:10.1056/NEJMra022710. PMID 12700377.
- ↑ 8.0 8.1 Fenner, Frank J.; Gibbs, E. Paul J.; Murphy, Frederick A.; Rott, Rudolph; Studdert, Michael J.; White, David O. (1993). Veterinary Virology (2nd ed.). Academic Press, Inc. ISBN 0-12-253056-X.
- ↑ Gilbert, David (2014). The Sanford guide to antimicrobial therapy 2014. Sperryville, Va: Antimicrobial Therapy. ISBN 978-1930808782.
- ↑ Lion T (2014). "Adenovirus infections in immunocompetent and immunocompromised patients". Clin Microbiol Rev. 27 (3): 441–62. doi:10.1128/CMR.00116-13. PMC 4135893. PMID 24982316.
- ↑ Gilbert, David (2014). The Sanford guide to antimicrobial therapy 2014. Sperryville, Va: Antimicrobial Therapy. ISBN 978-1930808782.