Trastuzumab: Difference between revisions
No edit summary |
No edit summary |
||
| (3 intermediate revisions by the same user not shown) | |||
| Line 1: | Line 1: | ||
{{DrugProjectFormSinglePage | {{DrugProjectFormSinglePage | ||
|authorTag={{SS}} | |authorTag={{SS}}; {{AV}} | ||
|genericName=Trastuzumab | |genericName=Trastuzumab | ||
|aOrAn=a | |aOrAn=a | ||
|drugClass=Monoclonal antibodies | |drugClass=[[Monoclonal antibodies]] | ||
|indicationType=treatment | |indicationType=treatment | ||
|indication=[[Adjuvant Breast Cancer]], [[Metastatic Breast Cancer]], [[Metastatic Gastric Cancer]] | |indication=[[Adjuvant Breast Cancer]], [[Metastatic Breast Cancer]], [[Metastatic Gastric Cancer]] | ||
|hasBlackBoxWarning=Yes | |hasBlackBoxWarning=Yes | ||
|adverseReactions=[[edema]], [[peripheral edema]],[[tachycardia]], [[rash]], weight decreased, abdominal | |adverseReactions=[[edema]], [[peripheral edema]],[[tachycardia]], [[rash]], [[weight loss|weight decreased]], [[pain|abdominal pain]],[[diarrhea]],[[anorexia|loss of appetite]], [[nausea]], [[stomatitis]], [[vomiting]], [[anemia]], [[neutropenia]], [[thrombocytopenia]], infectious disease, [[breast cancer]], [[arthralgia]], [[backache]], [[myalgia]], [[asthenia]], [[dizziness]], [[headache]], [[insomnia]], [[renal impairment]], [[cough]], [[dyspnea]], [[nasopharyngitis]], [[pharyngitis]], [[rhinitis]], [[upper respiratory infection]], [[fatigue]], [[fever]], inflammatory disease of mucous membrane, [[shivering]] | ||
|blackBoxWarningTitle=<span style="color:#FF0000;">WARNING: CARDIOMYOPATHY, INFUSION REACTIONS, EMBRYO-FETAL TOXICITY, and PULMONARY TOXICITY</span> | |blackBoxWarningTitle=<span style="color:#FF0000;">WARNING: CARDIOMYOPATHY, INFUSION REACTIONS, EMBRYO-FETAL TOXICITY, and PULMONARY TOXICITY</span> | ||
|blackBoxWarningBody=<i><span style="color:#FF0000;">Cardiomyopathy: Herceptin can result in sub-clinical and clinical cardiac failure manifesting as CHF, and decreased LVEF, with greatest risk when administered concurrently with anthracyclines. Evaluate cardiac function prior to and during treatment. Discontinue Herceptin for cardiomyopathy. | |blackBoxWarningBody=<i><span style="color:#FF0000;">Cardiomyopathy: Herceptin can result in sub-clinical and clinical cardiac failure manifesting as CHF, and decreased LVEF, with greatest risk when administered concurrently with anthracyclines. Evaluate cardiac function prior to and during treatment. Discontinue Herceptin for cardiomyopathy. | ||
Infusion reactions, Pulmonary toxicity: Discontinue Herceptin for anaphylaxis, angioedema, interstitial pneumonitis, or acute respiratory distress syndrome. | Infusion reactions, Pulmonary toxicity: Discontinue Herceptin for anaphylaxis, angioedema, interstitial pneumonitis, or acute respiratory distress syndrome. | ||
Embryo-Fetal Toxicity: Exposure to Herceptin during pregnancy can result in oligohydramnios, in some cases complicated by pulmonary hypoplasia and neonatal death.</span></i> | Embryo-Fetal Toxicity: Exposure to Herceptin during pregnancy can result in oligohydramnios, in some cases complicated by pulmonary hypoplasia and neonatal death.</span></i> | ||
|fdaLIADAdult====Recommended Doses and Schedules=== | |fdaLIADAdult= | ||
=====Recommended Doses and Schedules===== | |||
* Do not administer as an intravenous push or bolus. Do not mix Trastuzumab with other drugs. | * Do not administer as an intravenous push or bolus. Do not mix Trastuzumab with other drugs. | ||
* Do not substitute Trastuzumab for or with ado-trastuzumab emtansine. | * Do not substitute Trastuzumab for or with ado-trastuzumab emtansine. | ||
======Breast Cancer===== | |||
* Dosing information | * Dosing information | ||
| Line 22: | Line 24: | ||
* During and following [[paclitaxel]], [[docetaxel]], or [[docetaxel]]/[[carboplatin]]: | * During and following [[paclitaxel]], [[docetaxel]], or [[docetaxel]]/[[carboplatin]]: | ||
:* Initial dosage: '''4 mg/kg''' as an intravenous infusion over 90 minutes then at '''2 mg/kg''' as an intravenous infusion over 30 minutes weekly during chemotherapy for the first 12 weeks [[(paclitaxel]] or [[docetaxel]]) or 18 weeks ([[docetaxel]]/[[carboplatin]]). | :* Initial dosage: '''4 mg/kg''' as an intravenous infusion over 90 minutes then at '''2 mg/kg''' as an intravenous infusion over 30 minutes weekly during [[chemotherapy]] for the first 12 weeks [[(paclitaxel]] or [[docetaxel]]) or 18 weeks ([[docetaxel]]/[[carboplatin]]). | ||
* One week following the last weekly dose of Trastuzumab, administer Trastuzumab at 6 mg/kg as an intravenous infusion over 30–90 minutes every three weeks. | * One week following the last weekly dose of Trastuzumab, administer Trastuzumab at 6 mg/kg as an intravenous infusion over 30–90 minutes every three weeks. | ||
* As a single agent within three weeks following completion of multi-modality, anthracycline-based chemotherapy regimens: | * As a single agent within three weeks following completion of multi-modality, [[chemotherapy regimen|anthracycline-based chemotherapy regimens]]: | ||
* Extending adjuvant treatment beyond one year is not recommended. | * Extending adjuvant treatment beyond one year is not recommended. | ||
=====Metastatic Gastric Cancer===== | |||
* Dosing information | * Dosing information | ||
| Line 45: | Line 40: | ||
===Dose Modifications=== | ===Dose Modifications=== | ||
=====Infusion Reactions===== | |||
* Decrease the rate of infusion for mild or moderate infusion reactions | * Decrease the rate of infusion for mild or [[infusion reactions|moderate infusion reactions]] | ||
* Interrupt the infusion in patients with dyspnea or clinically significant hypotension | * Interrupt the infusion in patients with dyspnea or clinically significant [[hypotension]] | ||
* Discontinue Trastuzumab for severe or life-threatening infusion reactions. | * Discontinue Trastuzumab for severe or [[infusion reactions|life-threatening infusion reactions]]. | ||
=====[[Cardiomyopathy]]===== | |||
* Assess [[left ventricular ejection fraction]] ([[LVEF]]) prior to initiation of Trastuzumab and at regular intervals during treatment. Withhold Trastuzumab dosing for at least 4 weeks for either of the following: | * Assess [[left ventricular ejection fraction]] ([[LVEF]]) prior to initiation of Trastuzumab and at regular intervals during treatment. Withhold Trastuzumab dosing for at least 4 weeks for either of the following: | ||
| Line 57: | Line 52: | ||
:* [[LVEF]] below institutional limits of normal and ≥ 10% absolute decrease in [[LVEF]] from pretreatment values. | :* [[LVEF]] below institutional limits of normal and ≥ 10% absolute decrease in [[LVEF]] from pretreatment values. | ||
|offLabelAdultGuideSupport=There is limited information regarding <i>Off-Label Guideline-Supported Use</i> of Trastuzumab in adult patients. | |offLabelAdultGuideSupport=There is limited information regarding <i>Off-Label Guideline-Supported Use</i> of Trastuzumab in adult patients. | ||
|offLabelAdultNoGuideSupport= | |offLabelAdultNoGuideSupport= | ||
=====Carcinoma of prostate===== | |||
* Dosing information | * Dosing information | ||
:* ‘’‘(4 mg/kg) IV on day 1 of cycle 1, then 2 mg/kg IV on days 2, 9, and 16’‘<ref name="pmid11685733">{{cite journal| author=Small EJ, Bok R, Reese DM, Sudilovsky D, Frohlich M| title=Docetaxel, estramustine, plus trastuzumab in patients with metastatic androgen-independent prostate cancer. | journal=Semin Oncol | year= 2001 | volume= 28 | issue= 4 Suppl 15 | pages= 71-6 | pmid=11685733 | doi= | pmc= | url=http://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=11685733 }} </ref> | :* ‘’‘(4 mg/kg) IV on day 1 of cycle 1, then 2 mg/kg IV on days 2, 9, and 16’‘<ref name="pmid11685733">{{cite journal| author=Small EJ, Bok R, Reese DM, Sudilovsky D, Frohlich M| title=Docetaxel, estramustine, plus trastuzumab in patients with metastatic androgen-independent prostate cancer. | journal=Semin Oncol | year= 2001 | volume= 28 | issue= 4 Suppl 15 | pages= 71-6 | pmid=11685733 | doi= | pmc= | url=http://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=11685733 }} </ref> | ||
=====[[Malignant meningitis]]===== | |||
* Dosing information | * Dosing information | ||
:* '''individual doses ranged from 4 to 150 mg''' <ref name="pmid23588955">{{cite journal| author=Zagouri F, Sergentanis TN, Bartsch R, Berghoff AS, Chrysikos D, de Azambuja E et al.| title=Intrathecal administration of trastuzumab for the treatment of meningeal carcinomatosis in HER2-positive metastatic breast cancer: a systematic review and pooled analysis. | journal=Breast Cancer Res Treat | year= 2013 | volume= 139 | issue= 1 | pages= 13-22 | pmid=23588955 | doi=10.1007/s10549-013-2525-y | pmc= | url=http://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=23588955 }} </ref> | :* '''individual doses ranged from 4 to 150 mg''' <ref name="pmid23588955">{{cite journal| author=Zagouri F, Sergentanis TN, Bartsch R, Berghoff AS, Chrysikos D, de Azambuja E et al.| title=Intrathecal administration of trastuzumab for the treatment of meningeal carcinomatosis in HER2-positive metastatic breast cancer: a systematic review and pooled analysis. | journal=Breast Cancer Res Treat | year= 2013 | volume= 139 | issue= 1 | pages= 13-22 | pmid=23588955 | doi=10.1007/s10549-013-2525-y | pmc= | url=http://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=23588955 }} </ref> | ||
=====[[Non-small cell lung cancer]]===== | |||
* Dosing information | * Dosing information | ||
| Line 75: | Line 71: | ||
|offLabelPedNoGuideSupport=There is limited information regarding <i>Off-Label Non–Guideline-Supported Use</i> of Trastuzumab in pediatric patients. | |offLabelPedNoGuideSupport=There is limited information regarding <i>Off-Label Non–Guideline-Supported Use</i> of Trastuzumab in pediatric patients. | ||
|contraindications=None. | |contraindications=None. | ||
|warnings====Cardiomyopathy=== | |warnings | ||
====Cardiomyopathy=== | |||
Trastuzumab can cause [[left ventricular cardiac dysfunction]], [[arrhythmias]], [[hypertension]], disabling [[cardiac failure]], [[cardiomyopathy]], and cardiac death. Trastuzumab can also cause asymptomatic decline in [[left ventricular ejection fraction]] ([[LVEF]]). | *Trastuzumab can cause [[left ventricular cardiac dysfunction]], [[arrhythmias]], [[hypertension]], disabling [[cardiac failure]], [[cardiomyopathy]], and cardiac death. Trastuzumab can also cause asymptomatic decline in [[left ventricular ejection fraction]] ([[LVEF]]). | ||
There is a 4–6 fold increase in the incidence of symptomatic [[myocardial dysfunction]] among patients receiving Trastuzumab as a single agent or in combination therapy compared with those not receiving Trastuzumab. The highest absolute incidence occurs when Trastuzumab is administered with an [[anthracycline]]. | There is a 4–6 fold increase in the incidence of symptomatic [[myocardial dysfunction]] among patients receiving Trastuzumab as a single agent or in combination therapy compared with those not receiving Trastuzumab. The highest absolute incidence occurs when Trastuzumab is administered with an [[anthracycline]]. | ||
Withhold Trastuzumab for ≥ 16% absolute decrease in [[LVEF]] from pre-treatment values or an [[LVEF]] value below institutional limits of normal and ≥ 10% absolute decrease in [[LVEF]] from pretreatment values | Withhold Trastuzumab for ≥ 16% absolute decrease in [[LVEF]] from pre-treatment values or an [[LVEF]] value below institutional limits of normal and ≥ 10% absolute decrease in [[LVEF]] from pretreatment values . The safety of continuation or resumption of Trastuzumab in patients with Trastuzumab-induced [[left ventricular cardiac dysfunction]] has not been studied. | ||
=====Cardiac Monitoring===== | |||
Conduct thorough cardiac assessment, including history, physical examination, and determination of [[LVEF]] by echocardiogram or MUGA scan. The following schedule is recommended: | *Conduct thorough cardiac assessment, including history, physical examination, and determination of [[LVEF]] by [[echocardiogram]] or [[MUGA]] scan. The following schedule is recommended: | ||
* Baseline [[LVEF]] measurement immediately prior to initiation of Trastuzumab | * Baseline [[LVEF]] measurement immediately prior to initiation of Trastuzumab | ||
* [[LVEF]] measurements every 3 months during and upon completion of Trastuzumab | * [[LVEF]] measurements every 3 months during and upon completion of Trastuzumab | ||
* Repeat [[LVEF]] measurement at 4 week intervals if Trastuzumab is withheld for significant [[left ventricular cardiac dysfunction]] | * Repeat [[LVEF]] measurement at 4 week intervals if Trastuzumab is withheld for significant [[left ventricular cardiac dysfunction]] | ||
* [[LVEF]] measurements every 6 months for at least 2 years following completion of Trastuzumab as a component of adjuvant therapy. | * [[LVEF]] measurements every 6 months for at least 2 years following completion of Trastuzumab as a component of adjuvant therapy. | ||
In Study 1, 15% (158/1031) of patients discontinued Trastuzumab due to clinical evidence of [[myocardial dysfunction]] or significant decline in [[LVEF]] after a median follow-up duration of 8.7 years in the AC-TH arm. In Study 3 (one-year Trastuzumab treatment), the number of patients who discontinued Trastuzumab due to cardiac toxicity at 12.6 months median duration of follow-up was 2.6% (44/1678). In Study 4, a total of 2.9% (31/1056) patients in the TCH arm (1.5% during the chemotherapy phase and 1.4% during the monotherapy phase) and 5.7% (61/1068) patients in the AC-TH arm (1.5% during the chemotherapy phase and 4.2% during the monotherapy phase) discontinued Trastuzumab due to cardiac toxicity. | In Study 1, 15% (158/1031) of patients discontinued Trastuzumab due to clinical evidence of [[myocardial dysfunction]] or significant decline in [[LVEF]] after a median follow-up duration of 8.7 years in the AC-TH arm. In Study 3 (one-year Trastuzumab treatment), the number of patients who discontinued Trastuzumab due to cardiac toxicity at 12.6 months median duration of follow-up was 2.6% (44/1678). In Study 4, a total of 2.9% (31/1056) patients in the TCH arm (1.5% during the [[chemotherapy]] phase and 1.4% during the monotherapy phase) and 5.7% (61/1068) patients in the AC-TH arm (1.5% during the [[chemotherapy]] phase and 4.2% during the monotherapy phase) discontinued Trastuzumab due to cardiac toxicity. | ||
Among 64 patients receiving adjuvant chemotherapy (Studies 1 and 2) who developed [[congestive heart failure]], one patient died of [[cardiomyopathy]], one patient died suddenly without documented etiology and 33 patients were receiving cardiac medication at last follow-up. Approximately 24% of the surviving patients had recovery to a normal [[LVEF]] (defined as ≥50%) and no symptoms on continuing medical management at the time of last follow-up. Incidence of [[congestive heart failure]] is presented in Table 1. The safety of continuation or resumption of Trastuzumab in patients with Trastuzumab-induced [[left ventricular cardiac dysfunction]] has not been studied. | Among 64 patients receiving adjuvant [[chemotherapy]] (Studies 1 and 2) who developed [[congestive heart failure]], one patient died of [[cardiomyopathy]], one patient died suddenly without documented etiology and 33 patients were receiving cardiac medication at last follow-up. Approximately 24% of the surviving patients had recovery to a normal [[LVEF]] (defined as ≥50%) and no symptoms on continuing medical management at the time of last follow-up. Incidence of [[congestive heart failure]] is presented in Table 1. The safety of continuation or resumption of Trastuzumab in patients with Trastuzumab-induced [[left ventricular cardiac dysfunction]] has not been studied. | ||
[[File:Trastuzumab_warning_01.png|thumb|none|400px|This image is provided by the National Library of Medicine.]] | [[File:Trastuzumab_warning_01.png|thumb|none|400px|This image is provided by the National Library of Medicine.]] | ||
In Study 3 (one-year Trastuzumab treatment), at a median follow-up duration of 8 years, the incidence of severe CHF (NYHA III & IV) was 0.8%, and the rate of mild symptomatic and asymptomatic left ventricular dysfunction was 4.6%. | *In Study 3 (one-year Trastuzumab treatment), at a median follow-up duration of 8 years, the incidence of [[CHF|severe CHF]] ([[NYHA]] III & IV) was 0.8%, and the rate of mild symptomatic and asymptomatic left ventricular dysfunction was 4.6%. | ||
[[File:Trastuzumab_warning_02.png|thumb|none|400px|This image is provided by the National Library of Medicine.]] | [[File:Trastuzumab_warning_02.png|thumb|none|400px|This image is provided by the National Library of Medicine.]] | ||
In Study 4, the incidence of NCI-CTC Grade 3/4 [[cardiac ischemia]]/[[infarction]] was higher in the Trastuzumab containing regimens: (AC-TH: 0.3% (3/1068) and TCH 0.2% (2/1056)) as compared to none in AC-T. | *In Study 4, the incidence of NCI-CTC Grade 3/4 [[cardiac ischemia]]/[[infarction]] was higher in the Trastuzumab containing regimens: (AC-TH: 0.3% (3/1068) and TCH 0.2% (2/1056)) as compared to none in AC-T. | ||
===Infusion Reactions=== | ===Infusion Reactions=== | ||
Infusion reactions consist of a symptom complex characterized by fever and chills, and on occasion included nausea, vomiting, pain (in some cases at tumor sites), headache, dizziness, dyspnea, hypotension, rash, and asthenia. | *Infusion reactions consist of a symptom complex characterized by [[fever]] and [[chills]], and on occasion included [[nausea]], [[vomiting]], [[pain]] (in some cases at tumor sites), [[headache]], [[dizziness]], [[dyspnea]], [[hypotension]], [[rash]], and [[asthenia]]. | ||
In postmarketing reports, serious and fatal infusion reactions have been reported. Severe reactions which include bronchospasm, anaphylaxis, angioedema, hypoxia, and severe hypotension, were usually reported during or immediately following the initial infusion. However, the onset and clinical course were variable including progressive worsening, initial improvement followed by clinical deterioration, or delayed post-infusion events with rapid clinical deterioration. For fatal events, death occurred within hours to days following a serious infusion reaction. | In postmarketing reports, serious and fatal infusion reactions have been reported. Severe reactions which include [[bronchospasm]], [[anaphylaxis]], [[angioedema]], [[hypoxia]], and severe [[hypotension]], were usually reported during or immediately following the initial infusion. However, the onset and clinical course were variable including progressive worsening, initial improvement followed by clinical deterioration, or delayed post-infusion events with rapid clinical deterioration. For fatal events, death occurred within hours to days following a serious infusion reaction. | ||
Interrupt Trastuzumab infusion in all patients experiencing dyspnea, clinically significant hypotension, and intervention of medical therapy administered, which may include: epinephrine, corticosteroids, diphenhydramine, bronchodilators, and oxygen. Patients should be evaluated and carefully monitored until complete resolution of signs and symptoms. Permanent discontinuation should be strongly considered in all patients with severe infusion reactions. | Interrupt Trastuzumab infusion in all patients experiencing [[dyspnea]], clinically significant [[hypotension]], and intervention of medical therapy administered, which may include: [[epinephrine]], [[corticosteroids]], [[diphenhydramine]], [[bronchodilators]], and [[oxygen]]. Patients should be evaluated and carefully monitored until complete resolution of signs and symptoms. Permanent discontinuation should be strongly considered in all patients with severe [[infusion reactions]]. | ||
There are no data regarding the most appropriate method of identification of patients who may safely be retreated with Trastuzumab after experiencing a severe infusion reaction. Prior to resumption of Trastuzumab infusion, the majority of patients who experienced a severe infusion reaction were pre-medicated with antihistamines and/or corticosteroids. While some patients tolerated Trastuzumab infusions, others had recurrent severe infusion reactions despite pre-medications. | There are no data regarding the most appropriate method of identification of patients who may safely be retreated with Trastuzumab after experiencing a severe infusion reaction. Prior to resumption of Trastuzumab infusion, the majority of patients who experienced a severe infusion reaction were pre-medicated with [[antihistamines]] and/or [[corticosteroids]]. While some patients tolerated Trastuzumab infusions, others had recurrent severe infusion reactions despite pre-medications. | ||
===Embryo-Fetal Toxicity=== | ===Embryo-Fetal Toxicity=== | ||
Trastuzumab can cause fetal harm when administered to a pregnant woman. In post marketing reports, use of Trastuzumab during pregnancy resulted in cases of [[oligohydramnios]] and [[oligohydramnios]] sequence manifesting as [[pulmonary hypoplasia]], [[skeletal abnormalities]], and neonatal death. Advise women of the potential hazard to the fetus resulting from Trastuzumab exposure during pregnancy and provide contraception counseling to women of childbearing potential. | *Trastuzumab can cause fetal harm when administered to a pregnant woman. In post marketing reports, use of Trastuzumab during pregnancy resulted in cases of [[oligohydramnios]] and [[oligohydramnios]] sequence manifesting as [[pulmonary hypoplasia]], [[skeletal abnormalities]], and neonatal death. Advise women of the potential hazard to the fetus resulting from Trastuzumab exposure during [[pregnancy]] and provide [[contraception]] counseling to women of childbearing potential. | ||
===Pulmonary Toxicity=== | ===Pulmonary Toxicity=== | ||
Trastuzumab use can result in serious and fatal pulmonary toxicity. Pulmonary toxicity includes [[dyspnea]], [[interstitial pneumonitis]], [[pulmonary infiltrates]], [[pleural effusions]], non-cardiogenic [[pulmonary edema]], [[pulmonary insufficiency]] and [[hypoxia]], [[acute respiratory distress syndrome]], and [[pulmonary fibrosis]]. Such events can occur as sequelae of infusion reactions. Patients with symptomatic intrinsic lung disease or with extensive tumor involvement of the lungs, resulting in dyspnea at rest, appear to have more severe toxicity. | *Trastuzumab use can result in serious and fatal pulmonary toxicity. Pulmonary toxicity includes [[dyspnea]], [[interstitial pneumonitis]], [[pulmonary infiltrates]], [[pleural effusions]], non-cardiogenic [[pulmonary edema]], [[pulmonary insufficiency]] and [[hypoxia]], [[acute respiratory distress syndrome]], and [[pulmonary fibrosis]]. Such events can occur as sequelae of [[infusion reactions]]. Patients with symptomatic intrinsic lung disease or with extensive tumor involvement of the lungs, resulting in [[dyspnea]] at rest, appear to have more severe toxicity. | ||
===Exacerbation of Chemotherapy-Induced Neutropenia=== | ===Exacerbation of Chemotherapy-Induced Neutropenia=== | ||
In randomized, controlled clinical trials the per-patient incidences of NCI CTC Grade 3–4 neutropenia and of [[febrile neutropenia]] were higher in patients receiving Trastuzumab in combination with [[myelosuppressive]] chemotherapy as compared to those who received chemotherapy alone. The incidence of septic death was similar among patients who received Trastuzumab and those who did not. | *In randomized, controlled clinical trials the per-patient incidences of NCI CTC Grade 3–4 [[neutropenia]] and of [[febrile neutropenia]] were higher in patients receiving Trastuzumab in combination with [[myelosuppressive]] [[chemotherapy]] as compared to those who received [[chemotherapy]] alone. The incidence of septic death was similar among patients who received Trastuzumab and those who did not. | ||
===HER2 Testing=== | ===HER2 Testing=== | ||
Detection of HER2 protein overexpression is necessary for selection of patients appropriate for Trastuzumab therapy because these are the only patients studied and for whom benefit has been shown. Due to differences in tumor histopathology, use FDA-approved tests for the specific tumor type (breast or gastric/gastroesophageal adenocarcinoma) to assess HER2 protein overexpression and HER2 gene amplification. Tests should be performed by laboratories with demonstrated proficiency in the specific technology being utilized. Improper assay performance, including use of suboptimally fixed tissue, failure to utilize specified reagents, deviation from specific assay instructions, and failure to include appropriate controls for assay validation, can lead to unreliable results. | *Detection of [[HER2]] protein [[overexpression]] is necessary for selection of patients appropriate for Trastuzumab therapy because these are the only patients studied and for whom benefit has been shown. Due to differences in tumor histopathology, use FDA-approved tests for the specific tumor type (breast or gastric/gastroesophageal adenocarcinoma) to assess HER2 protein [[overexpression]] and [[HER2]] gene amplification. Tests should be performed by laboratories with demonstrated proficiency in the specific technology being utilized. Improper assay performance, including use of suboptimally fixed tissue, failure to utilize specified reagents, deviation from specific assay instructions, and failure to include appropriate controls for assay validation, can lead to unreliable results. | ||
Several FDA-approved commercial assays are available to aid in the selection of [[breast cancer]] and metastatic gastric cancer patients for Trastuzumab therapy. Users should refer to the package inserts of specific assay kits for information on the Intended Use, and the validation and performance of each assay. | Several FDA-approved commercial assays are available to aid in the selection of [[breast cancer]] and metastatic gastric cancer patients for Trastuzumab therapy. Users should refer to the package inserts of specific assay kits for information on the Intended Use, and the validation and performance of each assay. | ||
Limitations in assay precision make it inadvisable to rely on a single method to rule out potential Trastuzumab benefit. | Limitations in assay precision make it inadvisable to rely on a single method to rule out potential Trastuzumab benefit. | ||
Treatment outcomes for adjuvant [[breast cancer]] (Studies 2 and 3) and for metastatic [[breast cancer]] (Study 5) as a function of IHC and FISH testing are provided in Tables 8 and 10. | Treatment outcomes for adjuvant [[breast cancer]] (Studies 2 and 3) and for metastatic [[breast cancer]] (Study 5) as a function of [[IHC]] and [[FISH]] testing are provided in Tables 8 and 10. | ||
Assessment of HER2 protein overexpression and HER2 gene amplification in metastatic gastric cancer should be performed using FDA-approved tests specifically for gastric cancers due to differences in gastric vs. breast histopathology, including incomplete membrane staining and more frequent heterogeneous expression of HER2 seen in gastric cancers. Study 7 demonstrated that gene amplification and protein overexpression were not as well correlated as with [[breast cancer]]. Treatment outcomes for metastatic gastric cancer (Study 7), based on HER2 gene amplification (FISH) and HER2 protein overexpression (IHC) test results are provided in Table 12. | Assessment of [[HER2]] protein overexpression and HER2 gene amplification in [[gastric cancer|metastatic gastric cancer]] should be performed using FDA-approved tests specifically for gastric cancers due to differences in gastric vs. breast histopathology, including incomplete membrane staining and more frequent heterogeneous expression of [[HER2]] seen in gastric cancers. Study 7 demonstrated that gene amplification and protein overexpression were not as well correlated as with [[breast cancer]]. Treatment outcomes for metastatic gastric cancer (Study 7), based on HER2 gene amplification ([[FISH]]) and [[HER2]] protein overexpression ([[IHC]]) test results are provided in Table 12. | ||
|clinicalTrials=The most common adverse reactions in patients receiving Trastuzumab in the adjuvant and metastatic [[breast cancer]] setting are fever, [[nausea]], [[vomiting]], [[infusion reactions]], [[diarrhea]], [[infections]], increased cough, [[headache]], [[fatigue]], [[dyspnea]], [[rash]], [[neutropenia]], [[anemia]], and [[myalgia]]. Adverse reactions requiring interruption or discontinuation of Trastuzumab treatment include [[CHF]], significant decline in [[left ventricular cardiac function]], severe [[infusion reactions]], and [[pulmonary toxicity]]. | |clinicalTrials= | ||
In the metastatic gastric cancer setting, the most common adverse reactions (≥ 10%) that were increased (≥ 5% difference) in the Trastuzumab arm as compared to the chemotherapy alone arm were [[neutropenia]], [[diarrhea]], [[fatigue]], [[anemia]], [[stomatitis]], weight loss, [[upper respiratory tract infections]], [[fever]], [[thrombocytopenia]], mucosal inflammation, [[nasopharyngitis]], and [[dysgeusia]]. The most common adverse reactions which resulted in discontinuation of treatment on the Trastuzumab-containing arm in the absence of disease progression were infection, [[diarrhea]], and [[febrile neutropenia]]. | *The most common adverse reactions in patients receiving Trastuzumab in the adjuvant and metastatic [[breast cancer]] setting are [[fever]], [[nausea]], [[vomiting]], [[infusion reactions]], [[diarrhea]], [[infections]], increased [[cough]], [[headache]], [[fatigue]], [[dyspnea]], [[rash]], [[neutropenia]], [[anemia]], and [[myalgia]]. Adverse reactions requiring interruption or discontinuation of Trastuzumab treatment include [[CHF]], significant decline in [[left ventricular cardiac function]], severe [[infusion reactions]], and [[pulmonary toxicity]]. | ||
*In the [[gastric cancer|metastatic gastric cancer]] setting, the most common adverse reactions (≥ 10%) that were increased (≥ 5% difference) in the Trastuzumab arm as compared to the [[chemotherapy]] alone arm were [[neutropenia]], [[diarrhea]], [[fatigue]], [[anemia]], [[stomatitis]], [[weight loss]], [[upper respiratory tract infections]], [[fever]], [[thrombocytopenia]], mucosal inflammation, [[nasopharyngitis]], and [[dysgeusia]]. The most common adverse reactions which resulted in discontinuation of treatment on the Trastuzumab-containing arm in the absence of disease progression were [[infection]], [[diarrhea]], and [[febrile neutropenia]]. | |||
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice. | *Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice. | ||
Adjuvant Breast Cancer Studies | |||
The data below reflect exposure to one-year Trastuzumab therapy across three randomized, open-label studies, Studies 1, 2, and 3, with (n=3678) or without (n= 3363) trastuzumab in the adjuvant treatment of [[breast cancer]]. | *The data below reflect exposure to one-year Trastuzumab therapy across three randomized, open-label studies, Studies 1, 2, and 3, with (n=3678) or without (n= 3363) trastuzumab in the adjuvant treatment of [[breast cancer]]. | ||
The data summarized in Table 3 below, from Study 3, reflect exposure to Trastuzumab in 1678 patients; the median treatment duration was 51 weeks and median number of infusions was 18. Among the 3386 patients enrolled in the observation and one-year Trastuzumab arms of Study 3 at a median duration of follow-up of 12.6 months in the Trastuzumab arm, the median age was 49 years (range: 21 to 80 years), 83% of patients were Caucasian, and 13% were Asian. | The data summarized in Table 3 below, from Study 3, reflect exposure to Trastuzumab in 1678 patients; the median treatment duration was 51 weeks and median number of infusions was 18. Among the 3386 patients enrolled in the observation and one-year Trastuzumab arms of Study 3 at a median duration of follow-up of 12.6 months in the Trastuzumab arm, the median age was 49 years (range: 21 to 80 years), 83% of patients were Caucasian, and 13% were Asian. | ||
| Line 139: | Line 137: | ||
In Study 3, a comparison of 3-weekly Trastuzumab treatment for two years versus one year was also performed. The rate of asymptomatic cardiac dysfunction was increased in the 2-year Trastuzumab treatment arm (8.1% versus 4.6% in the one-year Trastuzumab treatment arm). More patients experienced at least one adverse reaction of grade 3 or higher in the 2-year Trastuzumab treatment arm (20.4%) compared with the one-year Trastuzumab treatment arm (16.3%). | In Study 3, a comparison of 3-weekly Trastuzumab treatment for two years versus one year was also performed. The rate of asymptomatic cardiac dysfunction was increased in the 2-year Trastuzumab treatment arm (8.1% versus 4.6% in the one-year Trastuzumab treatment arm). More patients experienced at least one adverse reaction of grade 3 or higher in the 2-year Trastuzumab treatment arm (20.4%) compared with the one-year Trastuzumab treatment arm (16.3%). | ||
The safety data from Studies 1 and 2 were obtained from 3655 patients, of whom 2000 received Trastuzumab; the median treatment duration was 51 weeks. The median age was 49 years (range: 24–80); 84% of patients were White, 7% Black, 4% Hispanic, and 3% Asian. | The safety data from Studies 1 and 2 were obtained from 3655 patients, of whom 2000 received Trastuzumab; the median treatment duration was 51 weeks. The median age was 49 years (range: 24–80); 84% of patients were White, 7% Black, 4% Hispanic, and 3% Asian. | ||
In Study 1, only Grade 3–5 adverse events, treatment-related Grade 2 events, and Grade 2–5 dyspnea were collected during and for up to 3 months following protocol-specified treatment. The following non-cardiac adverse reactions of Grade 2–5 occurred at an incidence of at least 2% greater among patients receiving Trastuzumab plus chemotherapy as compared to chemotherapy alone: [[fatigue]] (29.5% vs. 22.4%), [[infection]] (24.0% vs. 12.8%), [[hot flashes]] (17.1% vs. 15.0%), [[anemia]] (12.3% vs. 6.7%), [[dyspnea]] (11.8% vs. 4.6%), [[rash]]/[[desquamation]] (10.9% vs. 7.6%), [[leukopenia]] (10.5% vs. 8.4%), [[neutropenia]] (6.4% vs. 4.3%), [[headache]] (6.2% vs. 3.8%), pain (5.5% vs. 3.0%), [[edema]] (4.7% vs. 2.7%) and [[insomnia]] (4.3% vs. 1.5%). The majority of these events were Grade 2 in severity. | In Study 1, only Grade 3–5 adverse events, treatment-related Grade 2 events, and Grade 2–5 [[dyspnea]] were collected during and for up to 3 months following protocol-specified treatment. The following non-cardiac adverse reactions of Grade 2–5 occurred at an incidence of at least 2% greater among patients receiving Trastuzumab plus [[chemotherapy]] as compared to chemotherapy alone: [[fatigue]] (29.5% vs. 22.4%), [[infection]] (24.0% vs. 12.8%), [[hot flashes]] (17.1% vs. 15.0%), [[anemia]] (12.3% vs. 6.7%), [[dyspnea]] (11.8% vs. 4.6%), [[rash]]/[[desquamation]] (10.9% vs. 7.6%), [[leukopenia]] (10.5% vs. 8.4%), [[neutropenia]] (6.4% vs. 4.3%), [[headache]] (6.2% vs. 3.8%), pain (5.5% vs. 3.0%), [[edema]] (4.7% vs. 2.7%) and [[insomnia]] (4.3% vs. 1.5%). The majority of these events were Grade 2 in severity. | ||
In Study 2, data collection was limited to the following investigator-attributed treatment-related adverse reactions: NCI-CTC Grade 4 and 5 hematologic toxicities, Grade 3–5 non-hematologic toxicities, selected Grade 2–5 toxicities associated with [[taxanes]] ([[myalgia]], [[arthralgias]], nail changes, motor neuropathy, [[sensory neuropathy]]) and Grade 1–5 cardiac toxicities occurring during chemotherapy and/or Trastuzumab treatment. The following non-cardiac adverse reactions of Grade 2–5 occurred at an incidence of at least 2% greater among patients receiving Trastuzumab plus chemotherapy as compared to chemotherapy alone: [[arthralgia]] (12.2% vs. 9.1%), nail changes (11.5% vs.6.8%), [[dyspnea]] (2.4% vs. 0.2%), and [[diarrhea]] (2.2% vs. 0%). The majority of these events were Grade 2 in severity. | In Study 2, data collection was limited to the following investigator-attributed treatment-related adverse reactions: NCI-CTC Grade 4 and 5 hematologic toxicities, Grade 3–5 non-hematologic toxicities, selected Grade 2–5 toxicities associated with [[taxanes]] ([[myalgia]], [[arthralgias]], nail changes, motor neuropathy, [[sensory neuropathy]]) and Grade 1–5 cardiac toxicities occurring during [[chemotherapy]] and/or Trastuzumab treatment. The following non-cardiac adverse reactions of Grade 2–5 occurred at an incidence of at least 2% greater among patients receiving Trastuzumab plus [[chemotherapy]] as compared to [[chemotherapy]] alone: [[arthralgia]] (12.2% vs. 9.1%), nail changes (11.5% vs.6.8%), [[dyspnea]] (2.4% vs. 0.2%), and [[diarrhea]] (2.2% vs. 0%). The majority of these events were Grade 2 in severity. | ||
Safety data from Study 4 reflect exposure to Trastuzumab as part of an adjuvant treatment regimen from 2124 patients receiving at least one dose of study treatment [AC-TH: n = 1068; TCH: n = 1056]. The overall median treatment duration was 54 weeks in both the AC-TH and TCH arms. The median number of infusions was 26 in the AC-TH arm and 30 in the TCH arm, including weekly infusions during the chemotherapy phase and every three week dosing in the monotherapy period. Among these patients, the median age was 49 years (range 22 to 74 years). In Study 4, the toxicity profile was similar to that reported in Studies 1, 2, and 3 with the exception of a low incidence of CHF in the TCH arm. | Safety data from Study 4 reflect exposure to Trastuzumab as part of an adjuvant treatment regimen from 2124 patients receiving at least one dose of study treatment [AC-TH: n = 1068; TCH: n = 1056]. The overall median treatment duration was 54 weeks in both the AC-TH and TCH arms. The median number of infusions was 26 in the AC-TH arm and 30 in the TCH arm, including weekly infusions during the [[chemotherapy]] phase and every three week dosing in the monotherapy period. Among these patients, the median age was 49 years (range 22 to 74 years). In Study 4, the toxicity profile was similar to that reported in Studies 1, 2, and 3 with the exception of a low incidence of [[CHF]] in the TCH arm. | ||
<i>Metastatic [[Breast Cancer]] Studies</i> | <i>Metastatic [[Breast Cancer]] Studies</i> | ||
The data below reflect exposure to Trastuzumab in one randomized, open-label study, Study 5, of chemotherapy with (n=235) or without (n=234) trastuzumab in patients with metastatic [[breast cancer]], and one single-arm study (Study 6; n=222) in patients with | The data below reflect exposure to Trastuzumab in one randomized, open-label study, Study 5, of chemotherapy with (n=235) or without (n=234) trastuzumab in patients with metastatic [[breast cancer]], and one single-arm study (Study 6; n=222) in patients with [[breast cancer|metastatic breast cancer]]. Data in Table 4 are based on Studies 5 and 6. | ||
Among the 464 patients treated in Study 5, the median age was 52 years (range: 25–77 years). Eighty-nine percent were White, 5% Black, 1% Asian and 5% other racial/ethnic groups. All patients received 4 mg/kg initial dose of Trastuzumab followed by 2 mg/kg weekly. The percentages of patients who received Trastuzumab treatment for ≥ 6 months and ≥ 12 months were 58% and 9%, respectively. | Among the 464 patients treated in Study 5, the median age was 52 years (range: 25–77 years). Eighty-nine percent were White, 5% Black, 1% Asian and 5% other racial/ethnic groups. All patients received 4 mg/kg initial dose of Trastuzumab followed by 2 mg/kg weekly. The percentages of patients who received Trastuzumab treatment for ≥ 6 months and ≥ 12 months were 58% and 9%, respectively. | ||
Among the 352 patients treated in single agent studies (213 patients from Study 6), the median age was 50 years (range 28–86 years), 86% were White, 3% were Black, 3% were Asian, and 8% in other racial/ethnic groups. Most of the patients received 4 mg/kg initial dose of Trastuzumab followed by 2 mg/kg weekly. The percentages of patients who received Trastuzumab treatment for ≥ 6 months and ≥ 12 months were 31% and 16%, respectively. | Among the 352 patients treated in single agent studies (213 patients from Study 6), the median age was 50 years (range 28–86 years), 86% were White, 3% were Black, 3% were Asian, and 8% in other racial/ethnic groups. Most of the patients received 4 mg/kg initial dose of Trastuzumab followed by 2 mg/kg weekly. The percentages of patients who received Trastuzumab treatment for ≥ 6 months and ≥ 12 months were 31% and 16%, respectively. | ||
| Line 153: | Line 151: | ||
<i>Metastatic [[Gastric Cancer]]</i> | <i>Metastatic [[Gastric Cancer]]</i> | ||
The data below are based on the exposure of 294 patients to Trastuzumab in combination with a [[fluoropyrimidine]] ([[capecitabine]] or 5-FU) and cisplatin (Study 7). In the Trastuzumab plus chemotherapy arm, the initial dose of Trastuzumab 8 mg/kg was administered on Day 1 (prior to chemotherapy) followed by 6 mg/kg every 21 days until disease progression. [[Cisplatin]] was administered at 80 mg/m2 on Day 1 and the [[fluoropyrimidine]] was administered as either [[capecitabine]] 1000 mg/m2 orally twice a day on Days 1-14 or 5-fluorouracil 800 mg/m2/day as a continuous intravenous infusion Days 1 through 5. Chemotherapy was administered for six 21-day cycles. Median duration of Trastuzumab treatment was 21 weeks; median number of Trastuzumab infusions administered was eight. | The data below are based on the exposure of 294 patients to Trastuzumab in combination with a [[fluoropyrimidine]] ([[capecitabine]] or 5-FU) and [[cisplatin]] (Study 7). In the Trastuzumab plus [[chemotherapy]] arm, the initial dose of Trastuzumab 8 mg/kg was administered on Day 1 (prior to [[chemotherapy]]) followed by 6 mg/kg every 21 days until disease progression. [[Cisplatin]] was administered at 80 mg/m2 on Day 1 and the [[fluoropyrimidine]] was administered as either [[capecitabine]] 1000 mg/m2 orally twice a day on Days 1-14 or [[5-fluorouracil]] 800 mg/m2/day as a continuous intravenous infusion Days 1 through 5. [[Chemotherapy]] was administered for six 21-day cycles. Median duration of Trastuzumab treatment was 21 weeks; median number of Trastuzumab infusions administered was eight. | ||
[[File:Trastuzumab_adverse_03.png|thumb|none|400px|This image is provided by the National Library of Medicine.]] | [[File:Trastuzumab_adverse_03.png|thumb|none|400px|This image is provided by the National Library of Medicine.]] | ||
The following subsections provide additional detail regarding adverse reactions observed in clinical trials of adjuvant breast, metastatic [[breast cancer]], metastatic gastric cancer, or post-marketing experience. | The following subsections provide additional detail regarding adverse reactions observed in clinical trials of adjuvant breast, metastatic [[breast cancer]], [[gastric cancer|metastatic gastric cancer]], or post-marketing experience. | ||
<i>[[Cardiomyopathy]]</i> | <i>[[Cardiomyopathy]]</i> | ||
Serial measurement of cardiac function (LVEF) was obtained in clinical trials in the adjuvant treatment of [[breast cancer]]. In Study 3, the median duration of follow-up was 12.6 months (12.4 months in the observation arm; 12.6 months in the 1-year Trastuzumab arm); and in Studies 1 and 2, 7.9 years in the AC-T arm, 8.3 years in the AC-TH arm. In Studies 1 and 2, 6% of all randomized patients with post-AC LVEF evaluation were not permitted to initiate Trastuzumab following completion of AC chemotherapy due to cardiac dysfunction (LVEF < LLN or ≥ 16 point decline in LVEF from baseline to end of AC). Following initiation of Trastuzumab therapy, the incidence of new-onset dose-limiting myocardial dysfunction was higher among patients receiving Trastuzumab and [[paclitaxel]] as compared to those receiving paclitaxel alone in Studies 1 and 2, and in patients receiving one-year Trastuzumab monotherapy compared to observation in Study 3 (see Table 6, Figures 1 and 2). The per-patient incidence of new-onset cardiac dysfunction, as measured by LVEF, remained similar when compared to the analysis performed at a median follow-up of 2.0 years in the AC-TH arm. This analysis also showed evidence of reversibility of left ventricular dysfunction, with 64.5% of patients who experienced symptomatic CHF in the AC-TH group being asymptomatic at latest follow-up, and 90.3% having full or partial LVEF recovery. | Serial measurement of cardiac function ([[LVEF]]) was obtained in clinical trials in the adjuvant treatment of [[breast cancer]]. In Study 3, the median duration of follow-up was 12.6 months (12.4 months in the observation arm; 12.6 months in the 1-year Trastuzumab arm); and in Studies 1 and 2, 7.9 years in the AC-T arm, 8.3 years in the AC-TH arm. In Studies 1 and 2, 6% of all randomized patients with post-AC LVEF evaluation were not permitted to initiate Trastuzumab following completion of AC chemotherapy due to cardiac dysfunction ([[LVEF]] < LLN or ≥ 16 point decline in [[LVEF]] from baseline to end of AC). Following initiation of Trastuzumab therapy, the incidence of new-onset dose-limiting myocardial dysfunction was higher among patients receiving Trastuzumab and [[paclitaxel]] as compared to those receiving [[paclitaxel]] alone in Studies 1 and 2, and in patients receiving one-year Trastuzumab monotherapy compared to observation in Study 3 (see Table 6, Figures 1 and 2). The per-patient incidence of new-onset cardiac dysfunction, as measured by [[LVEF]], remained similar when compared to the analysis performed at a median follow-up of 2.0 years in the AC-TH arm. This analysis also showed evidence of reversibility of left ventricular dysfunction, with 64.5% of patients who experienced symptomatic [[CHF]] in the AC-TH group being asymptomatic at latest follow-up, and 90.3% having full or partial [[LVEF]] recovery. | ||
[[File:Trastuzumab_adverse_04.png|thumb|none|400px|This image is provided by the National Library of Medicine.]] | [[File:Trastuzumab_adverse_04.png|thumb|none|400px|This image is provided by the National Library of Medicine.]] | ||
| Line 167: | Line 165: | ||
'''Figure 1 ''' | '''Figure 1 ''' | ||
Studies 1 and 2: Cumulative Incidence of Time to First LVEF Decline of ≥ 10 Percentage Points from Baseline and to Below 50% with Death as a Competing Risk Event | Studies 1 and 2: Cumulative Incidence of Time to First [[LVEF]] Decline of ≥ 10 Percentage Points from Baseline and to Below 50% with Death as a Competing Risk Event | ||
[[File:Trastuzumab_adverse_05.png|thumb|none|400px|This image is provided by the National Library of Medicine.]] | [[File:Trastuzumab_adverse_05.png|thumb|none|400px|This image is provided by the National Library of Medicine.]] | ||
| Line 175: | Line 173: | ||
'''Figure 2 ''' | '''Figure 2 ''' | ||
Study 3: Cumulative Incidence of Time to First LVEF Decline of ≥ 10 Percentage Points from Baseline and to Below 50% with Death as a Competing Risk Event | Study 3: Cumulative Incidence of Time to First [[LVEF]] Decline of ≥ 10 Percentage Points from Baseline and to Below 50% with Death as a Competing Risk Event | ||
[[File:Trastuzumab_adverse_06.png|thumb|none|400px|This image is provided by the National Library of Medicine.]] | [[File:Trastuzumab_adverse_06.png|thumb|none|400px|This image is provided by the National Library of Medicine.]] | ||
| Line 183: | Line 181: | ||
'''Figure 3 ''' | '''Figure 3 ''' | ||
Study 4: Cumulative Incidence of Time to First LVEF Decline of ≥10 Percentage Points from Baseline and to Below 50% with Death as a Competing Risk Event | Study 4: Cumulative Incidence of Time to First [[LVEF]] Decline of ≥10 Percentage Points from Baseline and to Below 50% with Death as a Competing Risk Event | ||
[[File:Trastuzumab_adverse_07.png|thumb|none|400px|This image is provided by the National Library of Medicine.]] | [[File:Trastuzumab_adverse_07.png|thumb|none|400px|This image is provided by the National Library of Medicine.]] | ||
Time 0 is the date of randomization. | Time 0 is the date of randomization. | ||
The incidence of treatment emergent congestive heart failure among patients in the metastatic breast cancer trials was classified for severity using the New York Heart Association classification system (I–IV, where IV is the most severe level of cardiac failure) (see Table 2). In the metastatic breast cancer trials the probability of cardiac dysfunction was highest in patients who received Trastuzumab concurrently with anthracyclines. | The incidence of treatment emergent [[congestive heart failure]] among patients in the [[breast cancer|metastatic breast cancer]] trials was classified for severity using the [[New York Heart Association]] classification system (I–IV, where IV is the most severe level of cardiac failure) (see Table 2). In the [[breast cancer|metastatic breast cancer]] trials the probability of cardiac dysfunction was highest in patients who received Trastuzumab concurrently with [[anthracyclines]]. | ||
In Study 7, 5.0% of patients in the Trastuzumab plus chemotherapy arm compared to 1.1% of patients in the chemotherapy alone arm had LVEF value below 50% with a ≥ 10% absolute decrease in LVEF from pretreatment values. | In Study 7, 5.0% of patients in the Trastuzumab plus [[chemotherapy]] arm compared to 1.1% of patients in the chemotherapy alone arm had [[LVEF]] value below 50% with a ≥ 10% absolute decrease in [[LVEF]] from pretreatment values. | ||
<i>Infusion Reactions</i> | <i>Infusion Reactions</i> | ||
During the first infusion with Trastuzumab, the symptoms most commonly reported were chills and fever, occurring in approximately 40% of patients in clinical trials. Symptoms were treated with acetaminophen, diphenhydramine, and meperidine (with or without reduction in the rate of Trastuzumab infusion); permanent discontinuation of Trastuzumab for infusional toxicity was required in < 1% of patients. Other signs and/or symptoms may include nausea, vomiting, pain (in some cases at tumor sites), rigors, headache, dizziness, dyspnea, hypotension, elevated blood pressure, rash, and asthenia. Infusional toxicity occurred in 21% and 35% of patients, and was severe in 1.4% and 9% of patients, on second or subsequent Trastuzumab infusions administered as monotherapy or in combination with chemotherapy, respectively. In the post-marketing setting, severe infusion reactions, including hypersensitivity, anaphylaxis, and angioedema have been reported. | During the first infusion with Trastuzumab, the symptoms most commonly reported were chills and fever, occurring in approximately 40% of patients in clinical trials. Symptoms were treated with [[acetaminophen]], [[diphenhydramine]], and [[meperidine]] (with or without reduction in the rate of Trastuzumab infusion); permanent discontinuation of Trastuzumab for infusional toxicity was required in < 1% of patients. Other signs and/or symptoms may include [[nausea]], [[vomiting]], [[pain]] (in some cases at tumor sites), rigors, [[headache]], [[dizziness]], [[dyspnea]], [[hypotension]], [[Hypertension|elevated blood pressure]], [[rash]], and [[asthenia]]. Infusional toxicity occurred in 21% and 35% of patients, and was severe in 1.4% and 9% of patients, on second or subsequent Trastuzumab infusions administered as monotherapy or in combination with [[chemotherapy]], respectively. In the post-marketing setting, severe infusion reactions, including [[hypersensitivity]], [[anaphylaxis]], and [[angioedema]] have been reported. | ||
<i>[[Anemia]]</i> | <i>[[Anemia]]</i> | ||
In randomized controlled clinical trials, the overall incidence of anemia (30% vs. 21% [Study 5]), of selected NCI-CTC Grade 2–5 anemia (12.3% vs. 6.7% [Study 1]), and of anemia requiring transfusions (0.1% vs. 0 patients [Study 2]) were increased in patients receiving Trastuzumab and chemotherapy compared with those receiving chemotherapy alone. Following the administration of Trastuzumab as a single agent (Study 6), the incidence of NCI-CTC Grade 3 anemia was < 1%. In Study 7 (metastatic gastric cancer) on the Trastuzumab containing arm as compared to the chemotherapy alone arm the overall incidence of anemia was 28% compared 21% and of NCI CTC Grade 3/4 anemia was 12.2% compared to 10.3%. | In randomized controlled clinical trials, the overall incidence of [[anemia]] (30% vs. 21% [Study 5]), of selected NCI-CTC Grade 2–5 [[anemia]] (12.3% vs. 6.7% [Study 1]), and of [[anemia]] requiring transfusions (0.1% vs. 0 patients [Study 2]) were increased in patients receiving Trastuzumab and [[chemotherapy]] compared with those receiving [[chemotherapy]] alone. Following the administration of Trastuzumab as a single agent (Study 6), the incidence of NCI-CTC Grade 3 [[anemia]] was < 1%. In Study 7 (metastatic gastric cancer) on the Trastuzumab containing arm as compared to the [[chemotherapy]] alone arm the overall incidence of [[anemia]] was 28% compared 21% and of NCI CTC Grade 3/4 anemia was 12.2% compared to 10.3%. | ||
<i>[[Neutropenia]]</i> | <i>[[Neutropenia]]</i> | ||
In randomized controlled clinical trials in the adjuvant setting, the incidence of selected NCI-CTC Grade 4–5 neutropenia (1.7% vs. 0.8% [Study 2]) and of selected Grade 2–5 neutropenia (6.4% vs. 4.3% [Study 1]) were increased in patients receiving Trastuzumab and chemotherapy compared with those receiving chemotherapy alone. In a randomized, controlled trial in patients with metastatic breast cancer, the incidences of NCI-CTC Grade 3/4 neutropenia (32% vs. 22%) and of febrile neutropenia (23% vs. 17%) were also increased in patients randomized to Trastuzumab in combination with myelosuppressive chemotherapy as compared to chemotherapy alone. In Study 7 (metastatic gastric cancer) on the Trastuzumab containing arm as compared to the chemotherapy alone arm, the incidence of NCI CTC Grade 3/4 neutropenia was 36.8% compared to 28.9%; febrile neutropenia 5.1% compared to 2.8%. | In randomized controlled clinical trials in the adjuvant setting, the incidence of selected NCI-CTC Grade 4–5 [[neutropenia]] (1.7% vs. 0.8% [Study 2]) and of selected Grade 2–5 [[neutropenia]] (6.4% vs. 4.3% [Study 1]) were increased in patients receiving Trastuzumab and chemotherapy compared with those receiving chemotherapy alone. In a randomized, controlled trial in patients with metastatic breast cancer, the incidences of NCI-CTC Grade 3/4 [[neutropenia]] (32% vs. 22%) and of [[febrile neutropenia]] (23% vs. 17%) were also increased in patients randomized to Trastuzumab in combination with myelosuppressive [[chemotherapy]] as compared to [[chemotherapy]] alone. In Study 7 ([[gastric cancer|metastatic gastric cancer]]) on the Trastuzumab containing arm as compared to the [[chemotherapy]] alone arm, the incidence of NCI CTC Grade 3/4 [[neutropenia]] was 36.8% compared to 28.9%; [[febrile neutropenia]] 5.1% compared to 2.8%. | ||
<i>Infection</i> | <i>Infection</i> | ||
The overall incidences of infection (46% vs. 30% [Study 5]), of selected NCI-CTC Grade 2–5 infection/febrile neutropenia (24.3% vs. 13.4% [Study 1]) and of selected Grade 3–5 infection/febrile neutropenia (2.9% vs. 1.4%) [Study 2]), were higher in patients receiving Trastuzumab and chemotherapy compared with those receiving chemotherapy alone. The most common site of infections in the adjuvant setting involved the upper respiratory tract, skin, and urinary tract. | The overall incidences of infection (46% vs. 30% [Study 5]), of selected NCI-CTC Grade 2–5 infection/[[febrile neutropenia]] (24.3% vs. 13.4% [Study 1]) and of selected Grade 3–5 infection/febrile [[neutropenia]] (2.9% vs. 1.4%) [Study 2]), were higher in patients receiving Trastuzumab and [[chemotherapy]] compared with those receiving [[chemotherapy]] alone. The most common site of infections in the adjuvant setting involved the upper respiratory tract, skin, and urinary tract. | ||
In Study 4, the overall incidence of infection was higher with the addition of Trastuzumab to AC-T but not to TCH [44% (AC-TH), 37% (TCH), 38% (AC-T)]. The incidences of NCI-CTC Grade 3-4 infection were similar [25% (AC-TH), 21% (TCH), 23% (AC-T)] across the three arms. | In Study 4, the overall incidence of infection was higher with the addition of Trastuzumab to AC-T but not to TCH [44% (AC-TH), 37% (TCH), 38% (AC-T)]. The incidences of NCI-CTC Grade 3-4 infection were similar [25% (AC-TH), 21% (TCH), 23% (AC-T)] across the three arms. | ||
In a randomized, controlled trial in treatment of metastatic breast cancer, the reported incidence of febrile neutropenia was higher (23% vs. 17%) in patients receiving Trastuzumab in combination with myelosuppressive chemotherapy as compared to chemotherapy alone. | In a randomized, controlled trial in treatment of [[breast cancer|metastatic breast cancer]], the reported incidence of [[febrile neutropenia]] was higher (23% vs. 17%) in patients receiving Trastuzumab in combination with [[chemotherapy|myelosuppressive chemotherapy]] as compared to [[chemotherapy]] alone. | ||
<i>Pulmonary Toxicity</i> | <i>Pulmonary Toxicity</i> | ||
| Line 213: | Line 211: | ||
Adjuvant [[Breast Cancer]] | Adjuvant [[Breast Cancer]] | ||
Among women receiving adjuvant therapy for breast cancer, the incidence of selected NCI-CTC Grade 2–5 pulmonary toxicity (14.3% vs. 5.4% [Study 1]) and of selected NCI-CTC Grade 3–5 pulmonary toxicity and spontaneous reported Grade 2 dyspnea (3.4% vs. 0.9% [Study 2]) was higher in patients receiving Trastuzumab and chemotherapy compared with chemotherapy alone. The most common pulmonary toxicity was dyspnea (NCI-CTC Grade 2–5: 11.8% vs. 4.6% [Study 1]; NCI-CTC Grade 2–5: 2.4% vs. 0.2% [Study 2]). | Among women receiving adjuvant therapy for breast cancer, the incidence of selected NCI-CTC Grade 2–5 pulmonary toxicity (14.3% vs. 5.4% [Study 1]) and of selected NCI-CTC Grade 3–5 pulmonary toxicity and spontaneous reported Grade 2 [[dyspnea]] (3.4% vs. 0.9% [Study 2]) was higher in patients receiving Trastuzumab and chemotherapy compared with [[chemotherapy]] alone. The most common pulmonary toxicity was [[dyspnea]] (NCI-CTC Grade 2–5: 11.8% vs. 4.6% [Study 1]; NCI-CTC Grade 2–5: 2.4% vs. 0.2% [Study 2]). | ||
Pneumonitis/pulmonary infiltrates occurred in 0.7% of patients receiving Trastuzumab compared with 0.3% of those receiving chemotherapy alone. Fatal respiratory failure occurred in 3 patients receiving Trastuzumab, one as a component of multi-organ system failure, as compared to 1 patient receiving chemotherapy alone. | [[Pneumonitis]]/pulmonary infiltrates occurred in 0.7% of patients receiving Trastuzumab compared with 0.3% of those receiving [[chemotherapy]] alone. Fatal [[respiratory failure]] occurred in 3 patients receiving Trastuzumab, one as a component of [[MODI|multi-organ system failure]], as compared to 1 patient receiving chemotherapy alone. | ||
In Study 3, there were 4 cases of interstitial pneumonitis in the one-year Trastuzumab treatment arm compared to none in the observation arm at a median follow-up duration of 12.6 months. | In Study 3, there were 4 cases of interstitial pneumonitis in the one-year Trastuzumab treatment arm compared to none in the observation arm at a median follow-up duration of 12.6 months. | ||
Metastatic [[Breast Cancer]] | Metastatic [[Breast Cancer]] | ||
Among women receiving Trastuzumab for treatment of metastatic breast cancer, the incidence of pulmonary toxicity was also increased. Pulmonary adverse events have been reported in the post-marketing experience as part of the symptom complex of infusion reactions. Pulmonary events include bronchospasm, hypoxia, dyspnea, pulmonary infiltrates, pleural effusions, non-cardiogenic pulmonary edema, and acute respiratory distress syndrome | Among women receiving Trastuzumab for treatment of [[breast cancer|metastatic breast cancer]], the incidence of pulmonary toxicity was also increased. Pulmonary adverse events have been reported in the post-marketing experience as part of the symptom complex of infusion reactions. Pulmonary events include [[bronchospasm]], [[hypoxia]], [[dyspnea]], [[pulmonary infiltrates]], [[pleural effusions]], [[pulmonary edema|non-cardiogenic pulmonary edema]], and [[acute respiratory distress syndrome]]. | ||
Thrombosis/Embolism | Thrombosis/Embolism | ||
In 4 randomized, controlled clinical trials, the incidence of thrombotic adverse events was higher in patients receiving Trastuzumab and chemotherapy compared to chemotherapy alone in three studies (2.6% vs. 1.5% [Study 1], 2.5% and 3.7% vs. 2.2% [Study 4] and 2.1% vs. 0% [Study 5]). | In 4 randomized, controlled clinical trials, the incidence of thrombotic adverse events was higher in patients receiving Trastuzumab and [[chemotherapy]] compared to [[chemotherapy]] alone in three studies (2.6% vs. 1.5% [Study 1], 2.5% and 3.7% vs. 2.2% [Study 4] and 2.1% vs. 0% [Study 5]). | ||
[[Diarrhea]] | [[Diarrhea]] | ||
Among women receiving adjuvant therapy for breast cancer, the incidence of NCI-CTC Grade 2–5 diarrhea (6.7% vs. 5.4% [Study 1]) and of NCI-CTC Grade 3–5 diarrhea (2.2% vs. 0% [Study 2]), and of Grade 1–4 diarrhea (7% vs. 1% [Study 3; one-year Trastuzumab treatment at 12.6 months median duration of follow-up]) were higher in patients receiving Trastuzumab as compared to controls. In Study 4, the incidence of Grade 3–4 diarrhea was higher [5.7% AC-TH, 5.5% TCH vs. 3.0% AC-T] and of Grade 1–4 was higher [51% AC-TH, 63% TCH vs. 43% AC-T] among women receiving Trastuzumab. Of patients receiving Trastuzumab as a single agent for the treatment of metastatic breast cancer, 25% experienced [[diarrhea]]. An increased incidence of diarrhea was observed in patients receiving Trastuzumab in combination with chemotherapy for treatment of metastatic [[breast cancer]]. | Among women receiving adjuvant therapy for breast cancer, the incidence of NCI-CTC Grade 2–5 [[diarrhea]] (6.7% vs. 5.4% [Study 1]) and of NCI-CTC Grade 3–5 diarrhea (2.2% vs. 0% [Study 2]), and of Grade 1–4 diarrhea (7% vs. 1% [Study 3; one-year Trastuzumab treatment at 12.6 months median duration of follow-up]) were higher in patients receiving Trastuzumab as compared to controls. In Study 4, the incidence of Grade 3–4 diarrhea was higher [5.7% AC-TH, 5.5% TCH vs. 3.0% AC-T] and of Grade 1–4 was higher [51% AC-TH, 63% TCH vs. 43% AC-T] among women receiving Trastuzumab. Of patients receiving Trastuzumab as a single agent for the treatment of metastatic breast cancer, 25% experienced [[diarrhea]]. An increased incidence of diarrhea was observed in patients receiving Trastuzumab in combination with chemotherapy for treatment of metastatic [[breast cancer]]. | ||
Renal Toxicity | Renal Toxicity | ||
In Study 7 (metastatic gastric cancer) on the Trastuzumab-containing arm as compared to the chemotherapy alone arm the incidence of renal impairment was 18% compared to 14.5%. Severe (Grade 3/4) renal failure was 2.7% on the Trastuzumab-containing arm compared to 1.7% on the chemotherapy only arm. Treatment discontinuation for renal insufficiency/failure was 2% on the Trastuzumab-containing arm and 0.3% on the chemotherapy only arm. | In Study 7 (metastatic gastric cancer) on the Trastuzumab-containing arm as compared to the [[chemotherapy]] alone arm the incidence of renal impairment was 18% compared to 14.5%. Severe (Grade 3/4) renal failure was 2.7% on the Trastuzumab-containing arm compared to 1.7% on the [[chemotherapy]] only arm. Treatment discontinuation for [[renal insufficiency]]/failure was 2% on the Trastuzumab-containing arm and 0.3% on the [[chemotherapy]] only arm. | ||
In the postmarketing setting, rare cases of nephrotic syndrome with pathologic evidence of glomerulopathy have been reported. The time to onset ranged from 4 months to approximately 18 months from initiation of Trastuzumab therapy. Pathologic findings included [[membranous glomerulonephritis]], [[focal glomerulosclerosis]], and [[fibrillary glomerulonephritis]]. Complications included volume overload and [[congestive heart failure]]. | In the postmarketing setting, rare cases of [[nephrotic syndrome]] with pathologic evidence of [[glomerulopathy]] have been reported. The time to onset ranged from 4 months to approximately 18 months from initiation of Trastuzumab therapy. Pathologic findings included [[membranous glomerulonephritis]], [[focal glomerulosclerosis]], and [[fibrillary glomerulonephritis]]. Complications included volume overload and [[congestive heart failure]]. | ||
===Immunogenicity=== | ===Immunogenicity=== | ||
As with all therapeutic proteins, there is a potential for immunogenicity. Among 903 women with metastatic breast cancer, human anti-human antibody (HAHA) to Trastuzumab was detected in one patient using an enzyme-linked immunosorbent assay (ELISA). This patient did not experience an allergic reaction. Samples for assessment of HAHA were not collected in studies of adjuvant breast cancer. | As with all therapeutic proteins, there is a potential for [[immunogenicity]]. Among 903 women with [[breast cancer|metastatic breast cancer]], human anti-human antibody (HAHA) to Trastuzumab was detected in one patient using an [[ELISA|enzyme-linked immunosorbent assay (ELISA)]]. This patient did not experience an allergic reaction. Samples for assessment of HAHA were not collected in studies of [[breast cancer|adjuvant breast cancer]]. | ||
The incidence of antibody formation is highly dependent on the sensitivity and the specificity of the assay. Additionally, the observed incidence of antibody (including neutralizing antibody) positivity in an assay may be influenced by several factors including assay methodology, sample handling, timing of sample collection, concomitant medications, and underlying disease. For these reasons, comparison of the incidence of antibodies to Trastuzumab with the incidence of antibodies to other products may be misleading. | The incidence of antibody formation is highly dependent on the sensitivity and the specificity of the assay. Additionally, the observed incidence of antibody (including neutralizing antibody) positivity in an assay may be influenced by several factors including assay methodology, sample handling, timing of sample collection, concomitant medications, and underlying disease. For these reasons, comparison of the incidence of antibodies to Trastuzumab with the incidence of antibodies to other products may be misleading. | ||
|postmarketing=The following adverse reactions have been identified during post approval use of Trastuzumab. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure. | |postmarketing=The following adverse reactions have been identified during post approval use of Trastuzumab. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure. | ||
* Infusion reaction | * [[Infusion reaction]] | ||
* [[Oligohydramnios]] or [[oligohydramnios]] sequence, including [[pulmonary hypoplasia]], skeletal abnormalities, and neonatal death | * [[Oligohydramnios]] or [[oligohydramnios]] sequence, including [[pulmonary hypoplasia]], skeletal abnormalities, and neonatal death | ||
* [[Glomerulopathy]] | * [[Glomerulopathy]] | ||
|drugInteractions=In Study 5, the mean serum trough concentration of trastuzumab was consistently elevated approximately 1.5–fold, when administered in combination with [[paclitaxel]] as compared to trough concentrations of trastuzumab when administered in combination with an [[anthracycline]] and [[cyclophosphamide]]. | |drugInteractions=In Study 5, the mean serum trough concentration of trastuzumab was consistently elevated approximately 1.5–fold, when administered in combination with [[paclitaxel]] as compared to trough concentrations of trastuzumab when administered in combination with an [[anthracycline]] and [[cyclophosphamide]]. | ||
In other pharmacokinetic studies, where Trastuzumab was administered in combination with [[paclitaxel]], [[docetaxel]], [[carboplatin]], or [[doxorubicin]], Trastuzumab did not alter the plasma concentrations of these chemotherapeutic agents, or the metabolites that were analyzed. In a drug interaction substudy conducted in patients in Study 7, the pharmacokinetics of [[cisplatin]], [[capecitabine]] and their metabolites were not altered when administered in combination with Trastuzumab. | In other pharmacokinetic studies, where Trastuzumab was administered in combination with [[paclitaxel]], [[docetaxel]], [[carboplatin]], or [[doxorubicin]], Trastuzumab did not alter the plasma concentrations of these [[chemotherapeutic agents]], or the metabolites that were analyzed. In a drug interaction substudy conducted in patients in Study 7, the pharmacokinetics of [[cisplatin]], [[capecitabine]] and their metabolites were not altered when administered in combination with Trastuzumab. | ||
|FDAPregCat=D | |FDAPregCat=D | ||
|useInPregnancyFDA=Trastuzumab can cause fetal harm when administered to a pregnant woman. In post-marketing reports use of Trastuzumab during pregnancy resulted in cases of oligohydramnios and of oligohydramnios sequence, manifesting as pulmonary hypoplasia, skeletal abnormalities, and neonatal death. | |useInPregnancyFDA=Trastuzumab can cause fetal harm when administered to a pregnant woman. In post-marketing reports use of Trastuzumab during [[pregnancy]] resulted in cases of oligohydramnios and of [[oligohydramnios]] sequence, manifesting as [[pulmonary hypoplasia]], skeletal abnormalities, and neonatal death. | ||
These case reports described oligohydramnios in pregnant women who received Trastuzumab either alone or in combination with chemotherapy. In some case reports, amniotic fluid index increased after Trastuzumab was stopped. In one case, Trastuzumab therapy resumed after the amniotic fluid index improved, and oligohydramnios recurred. | These case reports described [[oligohydramnios]] in pregnant women who received Trastuzumab either alone or in combination with [[chemotherapy]]. In some case reports, [[amniotic fluid]] index increased after Trastuzumab was stopped. In one case, Trastuzumab therapy resumed after the amniotic fluid index improved, and [[oligohydramnios]] recurred. | ||
Monitor women exposed to Trastuzumab during pregnancy for oligohydramnios. If oligohydramnios occurs, perform fetal testing that is appropriate for gestational age and consistent with community standards of care. The efficacy of IV hydration in management of oligohydramnios due to Trastuzumab exposure is not known. | Monitor women exposed to Trastuzumab during pregnancy for [[oligohydramnios]]. If [[oligohydramnios]] occurs, perform fetal testing that is appropriate for gestational age and consistent with community standards of care. The efficacy of IV hydration in management of [[oligohydramnios]] due to Trastuzumab exposure is not known. | ||
Advise women of the potential hazard to the fetus resulting from Trastuzumab exposure during pregnancy. Encourage pregnant women with breast cancer who are using Trastuzumab to enroll in MotHER the Trastuzumab Pregnancy Registry: phone 1 800 690 6720 | Advise women of the potential hazard to the fetus resulting from Trastuzumab exposure during pregnancy. Encourage pregnant women with [[breast cancer]] who are using Trastuzumab to enroll in MotHER the Trastuzumab Pregnancy Registry: phone 1 800 690 6720. | ||
No teratogenic effects were observed in offspring from reproduction studies in cynomolgus monkeys at doses up to 25 times the recommended weekly human dose of 2 mg/kg trastuzumab. In mutant mice lacking HER2, embryos died in early gestation. Trastuzumab exposure was reported at delivery in offspring of cynomolgus monkeys treated during the early (Days 20–50 of gestation) or late (Days 120–150 of gestation) fetal development periods, at levels of 15 to 28% of the maternal blood levels. | No [[teratogenic]] effects were observed in offspring from reproduction studies in cynomolgus monkeys at doses up to 25 times the recommended weekly human dose of 2 mg/kg trastuzumab. In mutant mice lacking [[HER2]], embryos died in early gestation. Trastuzumab exposure was reported at delivery in offspring of cynomolgus monkeys treated during the early (Days 20–50 of gestation) or late (Days 120–150 of gestation) fetal development periods, at levels of 15 to 28% of the maternal blood levels. | ||
|useInNursing=It is not known whether Trastuzumab is excreted in human milk, but human IgG is excreted in human milk. Published data suggest that breast milk antibodies do not enter the neonatal and infant circulation in substantial amounts. | |useInNursing=It is not known whether Trastuzumab is excreted in human milk, but human [[IgG]] is excreted in human milk. Published data suggest that [[breast milk]] antibodies do not enter the neonatal and infant circulation in substantial amounts. | ||
Trastuzumab was present in the breast milk of lactating cynomolgus monkeys given 12.5 times the recommended weekly human dose of 2 mg/kg of Trastuzumab. Infant monkeys with detectable serum levels of trastuzumab did not have any adverse effects on growth or development from birth to 3 months of age; however, trastuzumab levels in animal breast milk may not accurately reflect human breast milk levels. | Trastuzumab was present in the breast milk of lactating cynomolgus monkeys given 12.5 times the recommended weekly human dose of 2 mg/kg of Trastuzumab. Infant monkeys with detectable serum levels of trastuzumab did not have any adverse effects on growth or development from birth to 3 months of age; however, trastuzumab levels in animal breast milk may not accurately reflect human [[breast milk]] levels. | ||
Because many drugs are secreted in human milk and because of the potential for serious adverse reactions in nursing infants from Trastuzumab, a decision should be made whether to discontinue nursing, or discontinue drug, taking into account the elimination half-life of trastuzumab and the importance of the drug to the mother. | Because many drugs are secreted in human milk and because of the potential for serious adverse reactions in nursing infants from Trastuzumab, a decision should be made whether to discontinue nursing, or discontinue drug, taking into account the elimination half-life of trastuzumab and the importance of the drug to the mother. | ||
|useInPed=The safety and effectiveness of Trastuzumab in pediatric patients has not been established. | |useInPed=The safety and effectiveness of Trastuzumab in pediatric patients has not been established. | ||
|useInGeri=Trastuzumab has been administered to 386 patients who were 65 years of age or over (253 in the adjuvant treatment and 133 in metastatic breast cancer treatment settings). The risk of cardiac dysfunction was increased in geriatric patients as compared to younger patients in both those receiving treatment for metastatic disease in Studies 5 and 6, or adjuvant therapy in Studies 1 and 2. Limitations in data collection and differences in study design of the 4 studies of Trastuzumab in adjuvant treatment of breast cancer preclude a determination of whether the toxicity profile of Trastuzumab in older patients is different from younger patients. The reported clinical experience is not adequate to determine whether the efficacy improvements (ORR, TTP, OS, DFS) of Trastuzumab treatment in older patients is different from that observed in patients <65 years of age for metastatic disease and adjuvant treatment. | |useInGeri=Trastuzumab has been administered to 386 patients who were 65 years of age or over (253 in the adjuvant treatment and 133 in metastatic [[breast cancer]] treatment settings). The risk of cardiac dysfunction was increased in geriatric patients as compared to younger patients in both those receiving treatment for metastatic disease in Studies 5 and 6, or adjuvant therapy in Studies 1 and 2. Limitations in data collection and differences in study design of the 4 studies of Trastuzumab in adjuvant treatment of breast cancer preclude a determination of whether the toxicity profile of Trastuzumab in older patients is different from younger patients. The reported clinical experience is not adequate to determine whether the efficacy improvements (ORR, TTP, OS, DFS) of Trastuzumab treatment in older patients is different from that observed in patients <65 years of age for metastatic disease and adjuvant treatment. | ||
In Study 7 (metastatic gastric cancer), of the 294 patients treated with Trastuzumab 108 (37%) were 65 years of age or older, while 13 (4.4%) were 75 and over. No overall differences in safety or effectiveness were observed. | In Study 7 ([[gastric cancer|metastatic gastric cancer]]), of the 294 patients treated with Trastuzumab 108 (37%) were 65 years of age or older, while 13 (4.4%) were 75 and over. No overall differences in safety or effectiveness were observed. | ||
|administration=To prevent medication errors, it is important to check the vial labels to ensure that the drug being prepared and administered is Trastuzumab and not ado-trastuzumab emtansine. | |administration=To prevent medication errors, it is important to check the vial labels to ensure that the drug being prepared and administered is Trastuzumab and not ado-trastuzumab emtansine. | ||
| Line 270: | Line 268: | ||
<i>Dilution</i> | <i>Dilution</i> | ||
* Determine the dose (mg) of Trastuzumab | * Determine the dose (mg) of Trastuzumab. Calculate the volume of the 21 mg/mL reconstituted Trastuzumab solution needed, withdraw this amount from the vial and add it to an infusion bag containing 250 mL of [[saline|0.9% Sodium Chloride]] Injection, USP. | ||
DO NOT USE DEXTROSE (5%) SOLUTION. | DO NOT USE DEXTROSE (5%) SOLUTION. | ||
* Gently invert the bag to mix the solution. | * Gently invert the bag to mix the solution. | ||
| Line 324: | Line 322: | ||
| molecular_weight = 145531.5 g/mol | | molecular_weight = 145531.5 g/mol | ||
}} | }} | ||
|mechAction=The HER2 (or c-erbB2) proto-oncogene encodes a transmembrane receptor protein of 185 kDa, which is structurally related to the epidermal growth factor receptor. Trastuzumab has been shown, in both in vitro assays and in animals, to inhibit the proliferation of human tumor cells that overexpress HER2. | |mechAction=The HER2 (or c-erbB2) proto-oncogene encodes a transmembrane receptor protein of 185 kDa, which is structurally related to the epidermal growth factor receptor. Trastuzumab has been shown, in both in vitro assays and in animals, to inhibit the proliferation of human tumor cells that overexpress [[HER2]]. | ||
Trastuzumab is a mediator of antibody-dependent cellular cytotoxicity (ADCC). In vitro, Trastuzumab-mediated ADCC has been shown to be preferentially exerted on HER2 overexpressing cancer cells compared with cancer cells that do not overexpress HER2. | Trastuzumab is a mediator of [[antibody-dependent cellular cytotoxicity]] (ADCC). In vitro, Trastuzumab-mediated ADCC has been shown to be preferentially exerted on HER2 overexpressing cancer cells compared with cancer cells that do not [[HER2|overexpress HER2]]. | ||
|structure=Trastuzumab is a humanized IgG1 kappa monoclonal antibody that selectively binds with high affinity to the extracellular domain of the human epidermal growth factor receptor 2 protein, HER2. Trastuzumab is produced by recombinant DNA technology in a mammalian cell (Chinese Hamster Ovary) culture containing the antibiotic gentamicin. Gentamicin is not detectable in the final product. | |structure=Trastuzumab is a humanized IgG1 kappa monoclonal antibody that selectively binds with high affinity to the extracellular domain of the [[EGF|human epidermal growth factor receptor]] 2 protein, [[HER2]]. Trastuzumab is produced by [[recombinant DNA technology]] in a mammalian cell (Chinese Hamster Ovary) culture containing the antibiotic [[gentamicin]]. [[Gentamicin]] is not detectable in the final product. | ||
Trastuzumab is a sterile, white to pale yellow, preservative-free lyophilized powder for intravenous administration. Each multi-use vial of Trastuzumab contains 440 mg trastuzumab, 400 mg α,α-trehalose dihydrate, 9.9 mg L-histidine HCl, 6.4 mg L-histidine, and 1.8 mg polysorbate 20, USP. Reconstitution with 20 mL of the appropriate diluent (BWFI or SWFI) yields a solution containing 21 mg/mL trastuzumab, at a pH of approximately 6. | Trastuzumab is a sterile, white to pale yellow, preservative-free lyophilized powder for intravenous administration. Each multi-use vial of Trastuzumab contains 440 mg trastuzumab, 400 mg α,α-trehalose dihydrate, 9.9 mg L-histidine HCl, 6.4 mg L-histidine, and 1.8 mg polysorbate 20, USP. Reconstitution with 20 mL of the appropriate diluent (BWFI or SWFI) yields a solution containing 21 mg/mL trastuzumab, at a pH of approximately 6. | ||
|PD=FDA Package Insert for Trastuzumab contains no information regarding Pharmacokinetics. | |PD=FDA Package Insert for Trastuzumab contains no information regarding [[Pharmacokinetics]]. | ||
|PK=The pharmacokinetics of trastuzumab were studied in women with metastatic breast cancer. Short duration intravenous infusions of 10 to 500 mg Trastuzumab once weekly demonstrated dose-dependent pharmacokinetics. Mean half-life increased and clearance decreased with increasing dose level. The half-life averaged 2 and 12 days at the 10 and 500 mg dose levels, respectively. The volume of distribution of trastuzumab was approximately that of serum volume (44 mL/kg). At the highest weekly dose studied (500 mg), mean peak serum concentrations were 377 mcg/mL. | |PK=The [[pharmacokinetics]] of trastuzumab were studied in women with [[breast cancer|metastatic breast cancer]]. Short duration intravenous infusions of 10 to 500 mg Trastuzumab once weekly demonstrated dose-dependent [[pharmacokinetics]]. Mean half-life increased and clearance decreased with increasing dose level. The half-life averaged 2 and 12 days at the 10 and 500 mg dose levels, respectively. The volume of distribution of trastuzumab was approximately that of serum volume (44 mL/kg). At the highest weekly dose studied (500 mg), mean peak serum concentrations were 377 mcg/mL. | ||
In studies using an initial dose of 4 mg/kg followed by a weekly dose of 2 mg/kg, a mean half-life of 6 days (range 1–32 days) was observed. Between weeks 16 and 32, trastuzumab serum concentrations reached a steady state with mean trough and peak concentrations of approximately 79 mcg/mL and 123 mcg/mL, respectively. | In studies using an initial dose of 4 mg/kg followed by a weekly dose of 2 mg/kg, a mean half-life of 6 days (range 1–32 days) was observed. Between weeks 16 and 32, trastuzumab serum concentrations reached a steady state with mean trough and peak concentrations of approximately 79 mcg/mL and 123 mcg/mL, respectively. | ||
In a study of women receiving adjuvant therapy for breast cancer, a mean half-life of trastuzumab of 16 days (range: 11–23 days) was observed after an initial dose of 8 mg/kg followed by a dose of 6 mg/kg every three weeks. Between weeks 6 and 37, trastuzumab serum concentrations reached a steady-state with mean trough and peak concentrations of 63 mcg/mL and 216 mcg/mL, respectively. | In a study of women receiving adjuvant therapy for [[breast cancer]], a mean half-life of trastuzumab of 16 days (range: 11–23 days) was observed after an initial dose of 8 mg/kg followed by a dose of 6 mg/kg every three weeks. Between weeks 6 and 37, trastuzumab serum concentrations reached a steady-state with mean trough and peak concentrations of 63 mcg/mL and 216 mcg/mL, respectively. | ||
In patients with metastatic gastric cancer (Study 7), mean serum trastuzumab trough concentrations at steady state were 24 to 63% lower as compared to the concentrations observed in patients with breast cancer receiving treatment for metastatic disease in combination with paclitaxel, as monotherapy for metastatic disease, or as adjuvant monotherapy. | In patients with [[gastric cancer|metastatic gastric cancer]] (Study 7), mean serum trastuzumab trough concentrations at steady state were 24 to 63% lower as compared to the concentrations observed in patients with breast cancer receiving treatment for metastatic disease in combination with [[paclitaxel]], as monotherapy for metastatic disease, or as adjuvant monotherapy. | ||
Sixty-four percent (286/447) of women with metastatic breast cancer had detectable circulating extracellular domain of the HER2 receptor (shed antigen), which ranged as high as 1880 ng/mL (median 11 ng/mL). Patients with higher baseline shed antigen levels were more likely to have lower serum trough concentrations. | Sixty-four percent (286/447) of women with [[breast cancer|metastatic breast cancer]] had detectable circulating extracellular domain of the [[HER2 receptor]] (shed antigen), which ranged as high as 1880 ng/mL (median 11 ng/mL). Patients with higher baseline shed antigen levels were more likely to have lower serum trough concentrations. | ||
Data suggest that the disposition of trastuzumab is not altered based on age or serum creatinine (≤2.0 mg creatinine/dL). | Data suggest that the disposition of trastuzumab is not altered based on age or [[serum creatinine]] (≤2.0 mg [[creatinine]]/dL). | ||
|nonClinToxic====Carcinogenesis, Mutagenesis, Impairment of Fertility=== | |nonClinToxic= | ||
===Carcinogenesis, Mutagenesis, Impairment of Fertility=== | |||
Trastuzumab has not been tested for carcinogenic potential. | Trastuzumab has not been tested for carcinogenic potential. | ||
No evidence of mutagenic activity was observed when trastuzumab was tested in the standard Ames bacterial and human peripheral blood lymphocyte mutagenicity assays, at concentrations of up to 5000 mcg/mL. In an in vivo micronucleus assay, no evidence of chromosomal damage to mouse bone marrow cells was observed following bolus intravenous doses of up to 118 mg/kg Trastuzumab. | No evidence of mutagenic activity was observed when trastuzumab was tested in the standard Ames bacterial and human peripheral blood lymphocyte [[mutagenicity]] assays, at concentrations of up to 5000 mcg/mL. In an in vivo [[micronucleus assay]], no evidence of chromosomal damage to mouse bone marrow cells was observed following bolus intravenous doses of up to 118 mg/kg Trastuzumab. | ||
A fertility study conducted in female cynomolgus monkeys at doses up to 25 times the weekly recommended human dose of 2 mg/kg trastuzumab and has revealed no evidence of impaired fertility, as measured by menstrual cycle duration and female sex hormone levels. Studies to evaluate the effects of trastuzumab on male fertility have not been conducted. | A [[fertility]] study conducted in female cynomolgus monkeys at doses up to 25 times the weekly recommended human dose of 2 mg/kg trastuzumab and has revealed no evidence of impaired [[fertility]], as measured by menstrual cycle duration and female sex hormone levels. Studies to evaluate the effects of trastuzumab on male [[fertility]] have not been conducted. | ||
===Animal Toxicology and/or Pharmacology=== | ===Animal Toxicology and/or Pharmacology=== | ||
| Line 345: | Line 344: | ||
<i>Reproductive Toxicology Studies</i> | <i>Reproductive Toxicology Studies</i> | ||
Reproductive toxicology studies have been conducted in cynomolgus monkeys at doses up to 25 times the weekly recommended human dose of 2 mg/kg Trastuzumab and have revealed no evidence of impaired fertility or harm to the fetus. However, HER2 protein expression is high in many embryonic tissues including cardiac and neural tissues; in mutant mice lacking HER2, embryos died in early gestation. Placental transfer of trastuzumab was detected at Caesarean section in offspring from pregnant cynomolgus monkeys dosed during the early (Days 20–50 of gestation) or late (Days 120–150 of gestation) fetal development periods. | Reproductive toxicology studies have been conducted in cynomolgus monkeys at doses up to 25 times the weekly recommended human dose of 2 mg/kg Trastuzumab and have revealed no evidence of impaired fertility or harm to the fetus. However, [[HER2 protein expression]] is high in many embryonic tissues including cardiac and neural tissues; in mutant mice lacking HER2, embryos died in early gestation. Placental transfer of trastuzumab was detected at Caesarean section in offspring from pregnant cynomolgus monkeys dosed during the early (Days 20–50 of gestation) or late (Days 120–150 of gestation) fetal development periods. | ||
|clinicalStudies====Adjuvant Breast Cancer=== | |clinicalStudies====Adjuvant Breast Cancer=== | ||
The safety and efficacy of Trastuzumab in women receiving adjuvant chemotherapy for HER2 overexpressing breast cancer, were evaluated in an integrated analysis of two randomized, open-label, clinical trials (Studies 1 and 2) with a total of 4063 women at the protocol-specified final overall survival analysis, a third randomized, open-label, clinical trial (Study 3) with a total of 3386 women at definitive Disease Free Survival analysis for one-year Trastuzumab treatment versus observation, and a fourth randomized, open-label clinical trial with a total of 3222 patients (Study 4). | The safety and efficacy of Trastuzumab in women receiving adjuvant [[chemotherapy]] for HER2 overexpressing [[breast cancer]], were evaluated in an integrated analysis of two randomized, open-label, clinical trials (Studies 1 and 2) with a total of 4063 women at the protocol-specified final overall survival analysis, a third randomized, open-label, clinical trial (Study 3) with a total of 3386 women at definitive Disease Free Survival analysis for one-year Trastuzumab treatment versus observation, and a fourth randomized, open-label clinical trial with a total of 3222 patients (Study 4). | ||
<i>Studies 1 and 2</i> | <i>Studies 1 and 2</i> | ||
In Studies 1 and 2, breast tumor specimens were required to show HER2 overexpression (3+ by IHC) or gene amplification (by FISH). HER2 testing was verified by a central laboratory prior to randomization (Study 2) or was required to be performed at a reference laboratory (Study 1). Patients with a history of active cardiac disease based on symptoms, abnormal electrocardiographic, radiologic, or left ventricular ejection fraction findings or uncontrolled hypertension (diastolic > 100 mmHg or systolic > 200 mmHg) were not eligible. | In Studies 1 and 2, breast tumor specimens were required to show [[HER2|HER2 overexpression]] (3+ by [[IHC]]) or gene amplification (by [[FISH]]). HER2 testing was verified by a central laboratory prior to randomization (Study 2) or was required to be performed at a reference laboratory (Study 1). Patients with a history of active cardiac disease based on symptoms, abnormal electrocardiographic, radiologic, or [[left ventricular ejection fraction]] findings or [[uncontrolled hypertension]] (diastolic > 100 mmHg or systolic > 200 mmHg) were not eligible. | ||
Patients were randomized (1:1) to receive doxorubicin and cyclophosphamide followed by paclitaxel (AC→paclitaxel) alone or paclitaxel plus Trastuzumab ( | Patients were randomized (1:1) to receive [[doxorubicin]] and [[cyclophosphamide]] followed by paclitaxel (AC→paclitaxel) alone or [[paclitaxel]] plus Trastuzumab (AC→[[paclitaxel]] + Trastuzumab). In both trials, patients received four 21-day cycles of [[doxorubicin]] 60 mg/m2 and [[cyclophosphamide]] 600 mg/m2. [[Paclitaxel]] was administered either weekly (80 mg/m2) or every 3 weeks (175 mg/m2) for a total of 12 weeks in Study 1; [[paclitaxel]] was administered only by the weekly schedule in Study 2. Trastuzumab was administered at 4 mg/kg on the day of initiation of paclitaxel and then at a dose of 2 mg/kg weekly for a total of 52 weeks. Trastuzumab treatment was permanently discontinued in patients who developed congestive heart failure, or persistent/recurrent [[LVEF]] decline. Radiation therapy, if administered, was initiated after the completion of chemotherapy. Patients with ER+ and/or PR+ tumors received hormonal therapy. The primary endpoint of the combined efficacy analysis was Disease-free survival (DFS), defined as the time from randomization to recurrence, occurrence of contralateral breast cancer, other second primary cancer, or death. The secondary endpoint was overall survival (OS). | ||
A total of 3752 patients were included in the joint efficacy analysis of the primary endpoint of DFS following a median follow-up of 2.0 years in the AC→paclitaxel + Trastuzumab arm. The pre-planned final OS analysis from the joint analysis included 4063 patients and was performed when 707 deaths had occurred after a median follow-up of 8.3 years in the AC→paclitaxel + Trastuzumab arm. The data from both arms in Study 1 and two of the three study arms in Study 2 were pooled for efficacy analyses. The patients included in the primary DFS analysis had a median age of 49 years (range, 22–80 years; 6% > 65 years), 84% were white, 7% black, 4% Hispanic, and 4% Asian/Pacific Islander. Disease characteristics included 90% infiltrating ductal histology, 38% T1, 91% nodal involvement, 27% intermediate and 66% high grade pathology, and 53% ER+ and/or PR+ tumors. Similar demographic and baseline characteristics were reported for the efficacy evaluable population, after 8.3 years of median follow-up in the AC→paclitaxel + Trastuzumab arm. | A total of 3752 patients were included in the joint efficacy analysis of the primary endpoint of DFS following a median follow-up of 2.0 years in the AC→paclitaxel + Trastuzumab arm. The pre-planned final OS analysis from the joint analysis included 4063 patients and was performed when 707 deaths had occurred after a median follow-up of 8.3 years in the AC→paclitaxel + Trastuzumab arm. The data from both arms in Study 1 and two of the three study arms in Study 2 were pooled for efficacy analyses. The patients included in the primary DFS analysis had a median age of 49 years (range, 22–80 years; 6% > 65 years), 84% were white, 7% black, 4% Hispanic, and 4% Asian/Pacific Islander. Disease characteristics included 90% infiltrating ductal histology, 38% T1, 91% nodal involvement, 27% intermediate and 66% high grade pathology, and 53% ER+ and/or PR+ tumors. Similar demographic and baseline characteristics were reported for the efficacy evaluable population, after 8.3 years of median follow-up in the AC→paclitaxel + Trastuzumab arm. | ||
<i>Study 3</i> | <i>Study 3</i> | ||
In Study 3, breast tumor specimens were required to show HER2 overexpression (3+ by IHC) or gene amplification (by FISH) as determined at a central laboratory. Patients with node-negative disease were required to have ≥ T1c primary tumor. Patients with a history of congestive heart failure or LVEF <55%, uncontrolled arrhythmias, angina requiring medication, clinically significant valvular heart disease, evidence of transmural infarction on ECG, poorly controlled hypertension (systolic > 180 mm Hg or diastolic > 100 mm Hg) were not eligible. | In Study 3, breast tumor specimens were required to show [[HER2|HER2 overexpression]] (3+ by IHC) or gene amplification (by FISH) as determined at a central laboratory. Patients with node-negative disease were required to have ≥ T1c primary tumor. Patients with a history of [[congestive heart failure]] or [[LVEF]] <55%, uncontrolled [[arrhythmias]], [[angina]] requiring medication, clinically significant valvular heart disease, evidence of transmural infarction on [[ECG]], poorly controlled [[hypertension]] (systolic > 180 mm Hg or diastolic > 100 mm Hg) were not eligible. | ||
Study 3 was designed to compare one and two years of three-weekly Trastuzumab treatment versus observation in patients with HER2 positive EBC following surgery, established chemotherapy and radiotherapy (if applicable). Patients were randomized (1:1:1) upon completion of definitive surgery, and at least four cycles of chemotherapy to receive no additional treatment, or one year of Trastuzumab treatment or two years of Trastuzumab treatment. Patients undergoing a lumpectomy had also completed standard radiotherapy. Patients with ER+ and/or PgR+ disease received systemic adjuvant hormonal therapy at investigator discretion. Trastuzumab was administered with an initial dose of 8 mg/kg followed by subsequent doses of 6 mg/kg once every three weeks. The main outcome measure was disease-free survival (DFS), defined as in Studies 1 and 2. | Study 3 was designed to compare one and two years of three-weekly Trastuzumab treatment versus observation in patients with HER2 positive EBC following surgery, established chemotherapy and radiotherapy (if applicable). Patients were randomized (1:1:1) upon completion of definitive surgery, and at least four cycles of [[chemotherapy]] to receive no additional treatment, or one year of Trastuzumab treatment or two years of Trastuzumab treatment. Patients undergoing a [[lumpectomy]] had also completed standard [[radiotherapy]]. Patients with ER+ and/or PgR+ disease received systemic adjuvant hormonal therapy at investigator discretion. Trastuzumab was administered with an initial dose of 8 mg/kg followed by subsequent doses of 6 mg/kg once every three weeks. The main outcome measure was disease-free survival (DFS), defined as in Studies 1 and 2. | ||
A protocol specified interim efficacy analysis comparing one-year Trastuzumab treatment to observation was performed at a median follow-up duration of 12.6 months in the Trastuzumab arm and formed the basis for the definitive DFS results from this study. Among the 3386 patients randomized to the observation (n =1693) and Trastuzumab one-year (n= 1693) treatment arms, the median age was 49 years (range 21–80), 83% were Caucasian, and 13% were Asian. Disease characteristics: 94% infiltrating ductal carcinoma, 50% ER+ and/or PgR+, 57% node positive, 32% node negative, and in 11% of patients, nodal status was not assessable due to prior neo-adjuvant chemotherapy. Ninety-six percent (1055/1098) of patients with node-negative disease had high risk features: among the 1098 patients with node-negative disease, 49% (543) were ER− and PgR−, and 47% (512) were ER and/or PgR + and had at least one of the following high risk features: pathological tumor size greater than 2 cm, Grade 2–3, or age < 35 years. Prior to randomization, 94% of patients had received anthracycline-based chemotherapy regimens. | A protocol specified interim efficacy analysis comparing one-year Trastuzumab treatment to observation was performed at a median follow-up duration of 12.6 months in the Trastuzumab arm and formed the basis for the definitive DFS results from this study. Among the 3386 patients randomized to the observation (n =1693) and Trastuzumab one-year (n= 1693) treatment arms, the median age was 49 years (range 21–80), 83% were Caucasian, and 13% were Asian. Disease characteristics: 94% infiltrating ductal carcinoma, 50% ER+ and/or PgR+, 57% node positive, 32% node negative, and in 11% of patients, nodal status was not assessable due to prior neo-adjuvant chemotherapy. Ninety-six percent (1055/1098) of patients with node-negative disease had high risk features: among the 1098 patients with node-negative disease, 49% (543) were ER− and PgR−, and 47% (512) were ER and/or PgR + and had at least one of the following high risk features: pathological tumor size greater than 2 cm, Grade 2–3, or age < 35 years. Prior to randomization, 94% of patients had received [[chemotherapy regimens|anthracycline-based chemotherapy regimens]]. | ||
After the definitive DFS results comparing observation to one-year Trastuzumab treatment were disclosed, a prospectively planned analysis that included comparison of one year versus two years of Trastuzumab treatment at a median follow-up duration of 8 years was performed. Based on this analysis, extending Trastuzumab treatment for a duration of two years did not show additional benefit over treatment for one year [Hazard Ratios of two-years Trastuzumab versus one-year Trastuzumab treatment in the intent to treat (ITT) population for Disease Free Survival (DFS) = 0.99 (95% CI: 0.87, 1.13), p-value = 0.90 and Overall Survival (OS) = 0.98 (0.83, 1.15); p-value= 0.78]. | After the definitive DFS results comparing observation to one-year Trastuzumab treatment were disclosed, a prospectively planned analysis that included comparison of one year versus two years of Trastuzumab treatment at a median follow-up duration of 8 years was performed. Based on this analysis, extending Trastuzumab treatment for a duration of two years did not show additional benefit over treatment for one year [Hazard Ratios of two-years Trastuzumab versus one-year Trastuzumab treatment in the intent to treat (ITT) population for Disease Free Survival (DFS) = 0.99 (95% CI: 0.87, 1.13), p-value = 0.90 and Overall Survival (OS) = 0.98 (0.83, 1.15); p-value= 0.78]. | ||
<i>Study 4</i> | <i>Study 4</i> | ||
In Study 4, breast tumor specimens were required to show HER2 gene amplification (FISH+ only) as determined at a central laboratory. Patients were required to have either node-positive disease, or node-negative disease with at least one of the following high-risk features: ER/PR-negative, tumor size > 2 cm, age < 35 years, or histologic and/or nuclear Grade 2 or 3. Patients with a history of CHF, myocardial infarction, Grade 3 or 4 cardiac arrhythmia, angina requiring medication, clinically significant valvular heart disease, poorly controlled hypertension (diastolic > 100 mmHg), any T4 or N2 or known N3 or M1 breast cancer were not eligible. | In Study 4, breast tumor specimens were required to show HER2 gene amplification (FISH+ only) as determined at a central laboratory. Patients were required to have either node-positive disease, or node-negative disease with at least one of the following high-risk features: ER/PR-negative, tumor size > 2 cm, age < 35 years, or histologic and/or nuclear Grade 2 or 3. Patients with a history of [[CHF]], [[myocardial infarction]], Grade 3 or 4 [[cardiac arrhythmia]], [[angina]] requiring medication, clinically significant valvular heart disease, poorly controlled hypertension (diastolic > 100 mmHg), any T4 or N2 or known N3 or M1 [[breast cancer]] were not eligible. | ||
Patients were randomized (1:1:1) to receive doxorubicin and cyclophosphamide followed by docetaxel (AC-T), doxorubicin and cyclophosphamide followed by docetaxel plus Trastuzumab (AC-TH), or docetaxel and carboplatin plus Trastuzumab (TCH). In both the AC-T and AC-TH arms, doxorubicin 60 mg/m2 and cyclophosphamide 600 mg/m2 were administered every 3 weeks for four cycles; docetaxel 100 mg/m 2 was administered every 3 weeks for four cycles. In the TCH arm, docetaxel 75 mg/m2 and carboplatin (at a target AUC of 6 mg/mL/min as a 30- to 60-minute infusion) were administered every 3 weeks for six cycles. Trastuzumab was administered weekly (initial dose of 4 mg/kg followed by weekly dose of 2 mg/kg) concurrently with either T or TC, and then every 3 weeks (6 mg/kg) as monotherapy for a total of 52 weeks. Radiation therapy, if administered, was initiated after completion of chemotherapy. Patients with ER+ and/or PR+ tumors received hormonal therapy. Disease-free survival (DFS) was the main outcome measure. | Patients were randomized (1:1:1) to receive [[doxorubicin]] and [[cyclophosphamide]] followed by [[docetaxel]] (AC-T), [[doxorubicin]] and [[cyclophosphamide]] followed by docetaxel plus Trastuzumab (AC-TH), or [[docetaxel]] and [[carboplatin]] plus Trastuzumab (TCH). In both the AC-T and AC-TH arms, [[doxorubicin]] 60 mg/m2 and [[cyclophosphamide]] 600 mg/m2 were administered every 3 weeks for four cycles; [[docetaxel]] 100 mg/m 2 was administered every 3 weeks for four cycles. In the TCH arm, [[docetaxel]] 75 mg/m2 and [[carboplatin]] (at a target AUC of 6 mg/mL/min as a 30- to 60-minute infusion) were administered every 3 weeks for six cycles. Trastuzumab was administered weekly (initial dose of 4 mg/kg followed by weekly dose of 2 mg/kg) concurrently with either T or TC, and then every 3 weeks (6 mg/kg) as [[monotherapy]] for a total of 52 weeks. Radiation therapy, if administered, was initiated after completion of [[chemotherapy]]. Patients with ER+ and/or PR+ tumors received hormonal therapy. Disease-free survival (DFS) was the main outcome measure. | ||
Among the 3222 patients randomized, the median age was 49 (range 22 to 74 years; 6% ≥ 65 years). Disease characteristics included 54% ER+ and/or PR+ and 71% node positive. Prior to randomization, all patients underwent primary surgery for breast cancer. | Among the 3222 patients randomized, the median age was 49 (range 22 to 74 years; 6% ≥ 65 years). Disease characteristics included 54% ER+ and/or PR+ and 71% node positive. Prior to randomization, all patients underwent primary surgery for [[breast cancer]]. | ||
The results for DFS for the integrated analysis of Studies 1 and 2, Study 3, and Study 4 and OS results for the integrated analysis of Studies 1 and 2, and Study 3 are presented in Table 7. For Studies 1 and 2, the duration of DFS following a median follow-up of 2.0 years in the AC→TH arm, is presented in Figure 4, and the duration of OS after a median follow-up of 8.3 years in the AC→TH arm is presented in Figure 5. The duration of DFS for Study 4 is presented in Figure 6. Across all four studies, at the time of definitive DFS analysis, there were insufficient numbers of patients within each of the following subgroups to determine if the treatment effect was different from that of the overall patient population: patients with low tumor grade, patients within specific ethnic/racial subgroups (Black, Hispanic, Asian/Pacific Islander patients), and patients > 65 years of age. For Studies 1 and 2, the OS hazard ratio was 0.64 (95% CI: 0.55, 0.74). At 8.3 years of median follow up [AC→TH], the survival rate was estimated to be 86.9% in the AC→TH arm and 79.4% in the AC→T arm. The final OS analysis results from Studies 1 and 2 indicate that OS benefit by age, hormone receptor status, number of positive lymph nodes, tumor size and grade, and surgery/radiation therapy, was consistent with the treatment effect in the overall population. In patients ≤ 50 years of age (n=2197), the OS hazard ratio was 0.65 (95% CI: 0.52, 0.81) and in patients > 50 years of age (n=1866), the OS hazard ratio was 0.63 (95% CI: 0.51, 0.78). In the subgroup of patients with hormone receptor-positive disease (ER-positive and/or PR-positive) (n=2223), the hazard ratio for OS was 0.63 (95% CI: 0.51, 0.78). In the subgroup of patients with hormone receptor-negative disease (ER-negative and PR-negative) (n=1830), the hazard ratio for OS was 0.64 (95% CI: 0.52, 0.80). In the subgroup of patients with tumor size ≤2 cm (n=1604), the hazard ratio for OS was 0.52 (95% CI: 0.39, 0.71). In the subgroup of patients with tumor size >2 cm (n=2448), the hazard ratio for OS was 0.67 (95% CI: 0.56, 0.80). | The results for DFS for the integrated analysis of Studies 1 and 2, Study 3, and Study 4 and OS results for the integrated analysis of Studies 1 and 2, and Study 3 are presented in Table 7. For Studies 1 and 2, the duration of DFS following a median follow-up of 2.0 years in the AC→TH arm, is presented in Figure 4, and the duration of OS after a median follow-up of 8.3 years in the AC→TH arm is presented in Figure 5. The duration of DFS for Study 4 is presented in Figure 6. Across all four studies, at the time of definitive DFS analysis, there were insufficient numbers of patients within each of the following subgroups to determine if the treatment effect was different from that of the overall patient population: patients with low tumor grade, patients within specific ethnic/racial subgroups (Black, Hispanic, Asian/Pacific Islander patients), and patients > 65 years of age. For Studies 1 and 2, the OS hazard ratio was 0.64 (95% CI: 0.55, 0.74). At 8.3 years of median follow up [AC→TH], the survival rate was estimated to be 86.9% in the AC→TH arm and 79.4% in the AC→T arm. The final OS analysis results from Studies 1 and 2 indicate that OS benefit by age, hormone receptor status, number of positive lymph nodes, tumor size and grade, and surgery/radiation therapy, was consistent with the treatment effect in the overall population. In patients ≤ 50 years of age (n=2197), the OS hazard ratio was 0.65 (95% CI: 0.52, 0.81) and in patients > 50 years of age (n=1866), the OS hazard ratio was 0.63 (95% CI: 0.51, 0.78). In the subgroup of patients with hormone receptor-positive disease (ER-positive and/or PR-positive) (n=2223), the hazard ratio for OS was 0.63 (95% CI: 0.51, 0.78). In the subgroup of patients with hormone receptor-negative disease (ER-negative and PR-negative) (n=1830), the hazard ratio for OS was 0.64 (95% CI: 0.52, 0.80). In the subgroup of patients with tumor size ≤2 cm (n=1604), the hazard ratio for OS was 0.52 (95% CI: 0.39, 0.71). In the subgroup of patients with tumor size >2 cm (n=2448), the hazard ratio for OS was 0.67 (95% CI: 0.56, 0.80). | ||
| Line 382: | Line 381: | ||
[[File:Trastuzumab_clincial studies_04.png|thumb|none|400px|This image is provided by the National Library of Medicine.]] | [[File:Trastuzumab_clincial studies_04.png|thumb|none|400px|This image is provided by the National Library of Medicine.]] | ||
Exploratory analyses of DFS as a function of HER2 overexpression or gene amplification were conducted for patients in Studies 2 and 3, where central laboratory testing data were available. The results are shown in Table 8. The number of events in Study 2 was small with the exception of the IHC 3+/FISH+ subgroup, which constituted 81% of those with data. Definitive conclusions cannot be drawn regarding efficacy within other subgroups due to the small number of events. The number of events in Study 3 was adequate to demonstrate significant effects on DFS in the IHC 3+/FISH unknown and the FISH +/IHC unknown subgroups. | Exploratory analyses of DFS as a function of [[HER2|HER2 overexpression]] or gene amplification were conducted for patients in Studies 2 and 3, where central laboratory testing data were available. The results are shown in Table 8. The number of events in Study 2 was small with the exception of the IHC 3+/FISH+ subgroup, which constituted 81% of those with data. Definitive conclusions cannot be drawn regarding efficacy within other subgroups due to the small number of events. The number of events in Study 3 was adequate to demonstrate significant effects on DFS in the IHC 3+/[[FISH]] unknown and the [[FISH]] +/IHC unknown subgroups. | ||
[[File:Trastuzumab_clincial studies_05.png|thumb|none|400px|This image is provided by the National Library of Medicine.]] | [[File:Trastuzumab_clincial studies_05.png|thumb|none|400px|This image is provided by the National Library of Medicine.]] | ||
| Line 388: | Line 387: | ||
===Metastatic Breast Cancer=== | ===Metastatic Breast Cancer=== | ||
The safety and efficacy of Trastuzumab in treatment of women with metastatic breast cancer were studied in a randomized, controlled clinical trial in combination with chemotherapy (Study 5, n=469 patients) and an open-label single agent clinical trial (Study 6, n=222 patients). Both trials studied patients with metastatic breast cancer whose tumors overexpress the HER2 protein. Patients were eligible if they had 2 or 3 levels of overexpression (based on a 0 to 3 scale) by immunohistochemical assessment of tumor tissue performed by a central testing lab. | The safety and efficacy of Trastuzumab in treatment of women with metastatic breast cancer were studied in a randomized, controlled clinical trial in combination with chemotherapy (Study 5, n=469 patients) and an open-label single agent clinical trial (Study 6, n=222 patients). Both trials studied patients with [[breast cancer|metastatic breast cancer]] whose tumors overexpress the [[HER2|HER2 protein]]. Patients were eligible if they had 2 or 3 levels of overexpression (based on a 0 to 3 scale) by immunohistochemical assessment of tumor tissue performed by a central testing lab. | ||
Previously Untreated Metastatic Breast Cancer (Study 5) | Previously Untreated Metastatic Breast Cancer (Study 5) | ||
Study 5 was a multicenter, randomized, open-label clinical trial conducted in 469 women with metastatic breast cancer who had not been previously treated with chemotherapy for metastatic disease. Tumor specimens were tested by IHC (Clinical Trial Assay, CTA) and scored as 0, 1+, 2+, or 3+, with 3+ indicating the strongest positivity. Only patients with 2+ or 3+ positive tumors were eligible (about 33% of those screened). Patients were randomized to receive chemotherapy alone or in combination with Trastuzumab given intravenously as a 4 mg/kg loading dose followed by weekly doses of Trastuzumab at 2 mg/kg. For those who had received prior anthracycline therapy in the adjuvant setting, chemotherapy consisted of paclitaxel (175 mg/m2 over 3 hours every 21 days for at least six cycles); for all other patients, chemotherapy consisted of anthracycline plus cyclophosphamide (AC: doxorubicin 60 mg/m2 or epirubicin 75 mg/m2 plus 600 mg/m2 cyclophosphamide every 21 days for six cycles). Sixty-five percent of patients randomized to receive chemotherapy alone in this study received Trastuzumab at the time of disease progression as part of a separate extension study. | Study 5 was a multicenter, randomized, open-label clinical trial conducted in 469 women with [[breast cancer|metastatic breast cancer]] who had not been previously treated with chemotherapy for metastatic disease. Tumor specimens were tested by [[IHC]] (Clinical Trial Assay, CTA) and scored as 0, 1+, 2+, or 3+, with 3+ indicating the strongest positivity. Only patients with 2+ or 3+ positive tumors were eligible (about 33% of those screened). Patients were randomized to receive [[chemotherapy]] alone or in combination with Trastuzumab given intravenously as a 4 mg/kg loading dose followed by weekly doses of Trastuzumab at 2 mg/kg. For those who had received prior [[anthracycline therapy]] in the adjuvant setting, [[chemotherapy]] consisted of paclitaxel (175 mg/m2 over 3 hours every 21 days for at least six cycles); for all other patients, [[chemotherapy]] consisted of [[anthracycline]] plus [[cyclophosphamide]] (AC: [[doxorubicin]] 60 mg/m2 or [[epirubicin]] 75 mg/m2 plus 600 mg/m2 [[cyclophosphamide]] every 21 days for six cycles). Sixty-five percent of patients randomized to receive chemotherapy alone in this study received Trastuzumab at the time of disease progression as part of a separate extension study. | ||
Based upon the determination by an independent response evaluation committee the patients randomized to Trastuzumab and chemotherapy experienced a significantly longer median time to disease progression, a higher overall response rate (ORR), and a longer median duration of response, as compared with patients randomized to chemotherapy alone. Patients randomized to Trastuzumab and chemotherapy also had a longer median survival (see Table 9). These treatment effects were observed both in patients who received Trastuzumab plus paclitaxel and in those who received Trastuzumab plus AC; however the magnitude of the effects was greater in the paclitaxel subgroup. | Based upon the determination by an independent response evaluation committee the patients randomized to Trastuzumab and [[chemotherapy]] experienced a significantly longer median time to disease progression, a higher overall response rate (ORR), and a longer median duration of response, as compared with patients randomized to chemotherapy alone. Patients randomized to Trastuzumab and [[chemotherapy]] also had a longer median survival (see Table 9). These treatment effects were observed both in patients who received Trastuzumab plus paclitaxel and in those who received Trastuzumab plus AC; however the magnitude of the effects was greater in the [[paclitaxel]] subgroup. | ||
[[File:Trastuzumab_clincial studies_06.png|thumb|none|400px|This image is provided by the National Library of Medicine.]] | [[File:Trastuzumab_clincial studies_06.png|thumb|none|400px|This image is provided by the National Library of Medicine.]] | ||
| Line 401: | Line 400: | ||
<i>Previously Treated Metastatic Breast Cancer (Study 6)</i> | <i>Previously Treated Metastatic Breast Cancer (Study 6)</i> | ||
Trastuzumab was studied as a single agent in a multicenter, open-label, single-arm clinical trial (Study 6) in patients with HER2 overexpressing metastatic breast cancer who had relapsed following one or two prior chemotherapy regimens for metastatic disease. Of 222 patients enrolled, 66% had received prior adjuvant chemotherapy, 68% had received two prior chemotherapy regimens for metastatic disease, and 25% had received prior myeloablative treatment with hematopoietic rescue. Patients were treated with a loading dose of 4 mg/kg IV followed by weekly doses of Trastuzumab at 2 mg/kg IV. | Trastuzumab was studied as a single agent in a multicenter, open-label, single-arm clinical trial (Study 6) in patients with [[HER2]] overexpressing [[breast cancer|metastatic breast cancer]] who had relapsed following one or two prior [[chemotherapy]] regimens for metastatic disease. Of 222 patients enrolled, 66% had received prior adjuvant chemotherapy, 68% had received two prior [[chemotherapy]] regimens for metastatic disease, and 25% had received prior myeloablative treatment with hematopoietic rescue. Patients were treated with a loading dose of 4 mg/kg IV followed by weekly doses of Trastuzumab at 2 mg/kg IV. | ||
The ORR (complete response+partial response), as determined by an independent Response Evaluation Committee, was 14%, with a 2% complete response rate and a 12% partial response rate. Complete responses were observed only in patients with disease limited to skin and lymph nodes. The overall response rate in patients whose tumors tested as CTA 3+ was 18% while in those that tested as CTA 2+, it was 6%. | The ORR (complete response+partial response), as determined by an independent Response Evaluation Committee, was 14%, with a 2% complete response rate and a 12% partial response rate. Complete responses were observed only in patients with disease limited to skin and lymph nodes. The overall response rate in patients whose tumors tested as CTA 3+ was 18% while in those that tested as CTA 2+, it was 6%. | ||
===Metastatic Gastric Cancer=== | ===Metastatic Gastric Cancer=== | ||
The safety and efficacy of Trastuzumab in combination with cisplatin and a fluoropyrimidine (capecitabine or 5-fluorouracil) were studied in patients previously untreated for metastatic gastric or gastroesophageal junction adenocarcinoma (Study 7). In this open-label, multi-center trial, 594 patients were randomized 1:1 to Trastuzumab in combination with cisplatin and a fluoropyrimidine (FC+H) or chemotherapy alone (FC). Randomization was stratified by extent of disease (metastatic vs. locally advanced), primary site (gastric vs. gastroesophageal junction), tumor measurability (yes vs. no), ECOG performance status (0,1 vs. 2), and fluoropyrimidine (capecitabine vs. 5-fluorouracil). All patients were either HER2 gene amplified (FISH+) or HER2 overexpressing (IHC 3+). Patients were also required to have adequate cardiac function (e.g., LVEF > 50%). | The safety and efficacy of Trastuzumab in combination with [[cisplatin]] and a [[fluoropyrimidine]] ([[capecitabine]] or [[5-fluorouracil]]) were studied in patients previously untreated for metastatic gastric or gastroesophageal junction [[adenocarcinoma]] (Study 7). In this open-label, multi-center trial, 594 patients were randomized 1:1 to Trastuzumab in combination with [[cisplatin]] and a [[fluoropyrimidine]] (FC+H) or [[chemotherapy]] alone (FC). Randomization was stratified by extent of disease (metastatic vs. locally advanced), primary site (gastric vs. gastroesophageal junction), tumor measurability (yes vs. no), ECOG performance status (0,1 vs. 2), and [[fluoropyrimidine]] ([[capecitabine]] vs. [[5-fluorouracil]]). All patients were either HER2 gene amplified ([[FISH|FISH+]]) or HER2 overexpressing (IHC 3+). Patients were also required to have adequate cardiac function (e.g., LVEF > 50%). | ||
On the Trastuzumab-containing arm, Trastuzumab was administered as an IV infusion at an initial dose of 8 mg/kg followed by 6 mg/kg every 3 weeks until disease progression. On both study arms cisplatin was administered at a dose of 80 mg/m2 Day 1 every 3 weeks for 6 cycles as a 2 hour IV infusion. On both study arms capecitabine was administered at 1000 mg/m2 dose orally twice daily (total daily dose 2000 mg/m2) for 14 days of each 21 day cycle for 6 cycles. Alternatively continuous intravenous infusion (CIV) 5-fluorouracil was administered at a dose of 800 mg/m2/day from Day 1 through Day 5 every three weeks for 6 cycles. | On the Trastuzumab-containing arm, Trastuzumab was administered as an IV infusion at an initial dose of 8 mg/kg followed by 6 mg/kg every 3 weeks until disease progression. On both study arms [[cisplatin]] was administered at a dose of 80 mg/m2 Day 1 every 3 weeks for 6 cycles as a 2 hour IV infusion. On both study arms capecitabine was administered at 1000 mg/m2 dose orally twice daily (total daily dose 2000 mg/m2) for 14 days of each 21 day cycle for 6 cycles. Alternatively continuous intravenous infusion (CIV) [[5-fluorouracil]] was administered at a dose of 800 mg/m2/day from Day 1 through Day 5 every three weeks for 6 cycles. | ||
The median age of the study population was 60 years (range: 21-83); 76% were male; 53% were Asian, 38% Caucasian, 5% Hispanic, 5% other racial/ethnic groups; 91% had ECOG PS of 0 or 1; 82% had primary gastric cancer and 18% had primary gastroesophageal adenocarcinoma. Of these patients, 23% had undergone prior gastrectomy, 7% had received prior neoadjuvant and/or adjuvant therapy, and 2% had received prior radiotherapy. | The median age of the study population was 60 years (range: 21-83); 76% were male; 53% were Asian, 38% Caucasian, 5% Hispanic, 5% other racial/ethnic groups; 91% had ECOG PS of 0 or 1; 82% had [[gastric cancer|primary gastric cancer]] and 18% had primary gastroesophageal [[adenocarcinoma]]. Of these patients, 23% had undergone prior [[gastrectomy]], 7% had received prior neoadjuvant and/or adjuvant therapy, and 2% had received prior radiotherapy. | ||
The main outcome measure of Study 7 was overall survival (OS), analyzed by the unstratified log-rank test. The final OS analysis based on 351 deaths was statistically significant (nominal significance level of 0.0193). An updated OS analysis was conducted at one year after the final analysis. The efficacy results of both the final and the updated analyses are summarized in Table 11 and Figure 7. | The main outcome measure of Study 7 was overall survival (OS), analyzed by the unstratified log-rank test. The final OS analysis based on 351 deaths was statistically significant (nominal significance level of 0.0193). An updated OS analysis was conducted at one year after the final analysis. The efficacy results of both the final and the updated analyses are summarized in Table 11 and Figure 7. | ||
[[File:Trastuzumab_clincial studies_09.png|thumb|none|400px|This image is provided by the National Library of Medicine.]] | [[File:Trastuzumab_clincial studies_09.png|thumb|none|400px|This image is provided by the National Library of Medicine.]] | ||
An exploratory analysis of OS in patients based on HER2 gene amplification (FISH) and protein overexpression (IHC) testing is summarized in Table 12. | An exploratory analysis of OS in patients based on [[HER2]] gene amplification ([[FISH]]) and protein overexpression (IHC) testing is summarized in Table 12. | ||
[[File:Trastuzumab_clincial studies_10.png|thumb|none|400px|This image is provided by the National Library of Medicine.]] | [[File:Trastuzumab_clincial studies_10.png|thumb|none|400px|This image is provided by the National Library of Medicine.]] | ||
|howSupplied=Trastuzumab is supplied in a multi-use vial containing 440 mg trastuzumab as a lyophilized sterile powder, under vacuum. Each carton contains one vial Trastuzumab® and one vial (20 mL) of Bacteriostatic Water for Injection (BWFI), USP, containing 1.1% benzyl alcohol as a preservative. NDC 50242-134-68. | |howSupplied=Trastuzumab is supplied in a multi-use vial containing 440 mg trastuzumab as a lyophilized sterile powder, under vacuum. Each carton contains one vial Trastuzumab® and one vial (20 mL) of [[Bacteriostatic]] Water for Injection (BWFI), USP, containing 1.1% benzyl alcohol as a preservative. NDC 50242-134-68. | ||
|storage=Vials of Trastuzumab are stable at 2–8°C (36–46°F) prior to reconstitution. | |storage=Vials of Trastuzumab are stable at 2–8°C (36–46°F) prior to reconstitution. | ||
Do not use beyond the expiration date stamped on the vial. A vial of Trastuzumab reconstituted with BWFI, as supplied, is stable for 28 days after reconstitution when stored refrigerated at 2–8°C (36–46°F). Discard any remaining multi-dose reconstituted solution after 28 days. A vial of Trastuzumab reconstituted with unpreserved SWFI (not supplied) should be used immediately and any unused portion discarded. Do Not Freeze Trastuzumab following reconstitution or dilution. | Do not use beyond the expiration date stamped on the vial. A vial of Trastuzumab reconstituted with BWFI, as supplied, is stable for 28 days after reconstitution when stored refrigerated at 2–8°C (36–46°F). Discard any remaining multi-dose reconstituted solution after 28 days. A vial of Trastuzumab reconstituted with unpreserved SWFI (not supplied) should be used immediately and any unused portion discarded. Do Not Freeze Trastuzumab following reconstitution or dilution. | ||
The solution of Trastuzumab for infusion diluted in polyvinylchloride or polyethylene bags containing 0.9% Sodium Chloride Injection, USP, should be stored at 2–8°C (36–46°F) for no more than 24 hours prior to use. | The solution of Trastuzumab for infusion diluted in [[polyvinylchloride]] or polyethylene bags containing 0.9% Sodium Chloride Injection, USP, should be stored at 2–8°C (36–46°F) for no more than 24 hours prior to use. | ||
|fdaPatientInfo=* Advise patients to contact a health care professional immediately for any of the following: new onset or worsening shortness of breath, cough, swelling of the ankles/legs, swelling of the face, palpitations, weight gain of more than 5 pounds in 24 hours, dizziness or loss of consciousness | |fdaPatientInfo=* Advise patients to contact a health care professional immediately for any of the following: new onset or worsening [[shortness of breath]], [[cough]], swelling of the ankles/legs, swelling of the face, [[palpitations]], [[weight gain]] of more than 5 pounds in 24 hours, [[dizziness]] or [[loss of consciousness]] | ||
* Advise pregnant women and women of childbearing potential that Trastuzumab exposure can result in fetal harm | * Advise pregnant women and women of childbearing potential that Trastuzumab exposure can result in fetal harm | ||
* Advise women of childbearing potential to use effective contraceptive methods during treatment and for a minimum of six months following Trastuzumab | * Advise women of childbearing potential to use effective contraceptive methods during treatment and for a minimum of six months following Trastuzumab | ||
* Advise nursing mothers treated with Trastuzumab to discontinue nursing or discontinue Trastuzumab, taking into account the importance of the drug to the mother | * Advise nursing mothers treated with Trastuzumab to discontinue nursing or discontinue Trastuzumab, taking into account the importance of the drug to the mother | ||
* Encourage women who are exposed to Trastuzumab during pregnancy to enroll in MotHER the Trastuzumab Pregnancy Registry (1-800-690-6720) | * Encourage women who are exposed to Trastuzumab during [[pregnancy]] to enroll in MotHER the Trastuzumab Pregnancy Registry (1-800-690-6720) | ||
|alcohol=Alcohol-Trastuzumab interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication. | |alcohol=Alcohol-Trastuzumab interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication. | ||
|brandNames=*[[Herceptin]] | |brandNames=*[[Herceptin]] | ||
Latest revision as of 16:02, 23 March 2015
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Sheng Shi, M.D. [2]; Aparna Vuppala, M.B.B.S. [3]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Black Box Warning
|
WARNING: CARDIOMYOPATHY, INFUSION REACTIONS, EMBRYO-FETAL TOXICITY, and PULMONARY TOXICITY
See full prescribing information for complete Boxed Warning.
Cardiomyopathy: Herceptin can result in sub-clinical and clinical cardiac failure manifesting as CHF, and decreased LVEF, with greatest risk when administered concurrently with anthracyclines. Evaluate cardiac function prior to and during treatment. Discontinue Herceptin for cardiomyopathy.
Infusion reactions, Pulmonary toxicity: Discontinue Herceptin for anaphylaxis, angioedema, interstitial pneumonitis, or acute respiratory distress syndrome. Embryo-Fetal Toxicity: Exposure to Herceptin during pregnancy can result in oligohydramnios, in some cases complicated by pulmonary hypoplasia and neonatal death. |
Overview
Trastuzumab is a Monoclonal antibodies that is FDA approved for the treatment of Adjuvant Breast Cancer, Metastatic Breast Cancer, Metastatic Gastric Cancer. There is a Black Box Warning for this drug as shown here. Common adverse reactions include edema, peripheral edema,tachycardia, rash, weight decreased, abdominal pain,diarrhea,loss of appetite, nausea, stomatitis, vomiting, anemia, neutropenia, thrombocytopenia, infectious disease, breast cancer, arthralgia, backache, myalgia, asthenia, dizziness, headache, insomnia, renal impairment, cough, dyspnea, nasopharyngitis, pharyngitis, rhinitis, upper respiratory infection, fatigue, fever, inflammatory disease of mucous membrane, shivering.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Recommended Doses and Schedules
- Do not administer as an intravenous push or bolus. Do not mix Trastuzumab with other drugs.
- Do not substitute Trastuzumab for or with ado-trastuzumab emtansine.
=Breast Cancer
- Dosing information
- Administer according to one of the following doses and schedules for a total of 52 weeks of Trastuzumab therapy:
- During and following paclitaxel, docetaxel, or docetaxel/carboplatin:
- Initial dosage: 4 mg/kg as an intravenous infusion over 90 minutes then at 2 mg/kg as an intravenous infusion over 30 minutes weekly during chemotherapy for the first 12 weeks (paclitaxel or docetaxel) or 18 weeks (docetaxel/carboplatin).
- One week following the last weekly dose of Trastuzumab, administer Trastuzumab at 6 mg/kg as an intravenous infusion over 30–90 minutes every three weeks.
- As a single agent within three weeks following completion of multi-modality, anthracycline-based chemotherapy regimens:
- Extending adjuvant treatment beyond one year is not recommended.
Metastatic Gastric Cancer
- Dosing information
- Initial dosage: 8 mg/kg as a 90 minute intravenous infusion
- Subsequent dosage: 6 mg/kg as an intravenous infusion over 30-90 minutes every three weeks until disease progression
Dose Modifications
Infusion Reactions
- Decrease the rate of infusion for mild or moderate infusion reactions
- Interrupt the infusion in patients with dyspnea or clinically significant hypotension
- Discontinue Trastuzumab for severe or life-threatening infusion reactions.
Cardiomyopathy
- Assess left ventricular ejection fraction (LVEF) prior to initiation of Trastuzumab and at regular intervals during treatment. Withhold Trastuzumab dosing for at least 4 weeks for either of the following:
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Trastuzumab in adult patients.
Non–Guideline-Supported Use
Carcinoma of prostate
- Dosing information
- ‘’‘(4 mg/kg) IV on day 1 of cycle 1, then 2 mg/kg IV on days 2, 9, and 16’‘[1]
Malignant meningitis
- Dosing information
- individual doses ranged from 4 to 150 mg [2]
Non-small cell lung cancer
- Dosing information
- 4 mg/kg is administered IV on day 1, then 2 mg/kg IV weekly thereafter[3]
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
The safety and effectiveness of Trastuzumab in pediatric patients has not been established.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Trastuzumab in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Trastuzumab in pediatric patients.
Contraindications
None.
Warnings
|
WARNING: CARDIOMYOPATHY, INFUSION REACTIONS, EMBRYO-FETAL TOXICITY, and PULMONARY TOXICITY
See full prescribing information for complete Boxed Warning.
Cardiomyopathy: Herceptin can result in sub-clinical and clinical cardiac failure manifesting as CHF, and decreased LVEF, with greatest risk when administered concurrently with anthracyclines. Evaluate cardiac function prior to and during treatment. Discontinue Herceptin for cardiomyopathy.
Infusion reactions, Pulmonary toxicity: Discontinue Herceptin for anaphylaxis, angioedema, interstitial pneumonitis, or acute respiratory distress syndrome. Embryo-Fetal Toxicity: Exposure to Herceptin during pregnancy can result in oligohydramnios, in some cases complicated by pulmonary hypoplasia and neonatal death. |
There is limited information regarding Trastuzumab Warnings' in the drug label.
Adverse Reactions
Clinical Trials Experience
- The most common adverse reactions in patients receiving Trastuzumab in the adjuvant and metastatic breast cancer setting are fever, nausea, vomiting, infusion reactions, diarrhea, infections, increased cough, headache, fatigue, dyspnea, rash, neutropenia, anemia, and myalgia. Adverse reactions requiring interruption or discontinuation of Trastuzumab treatment include CHF, significant decline in left ventricular cardiac function, severe infusion reactions, and pulmonary toxicity.
- In the metastatic gastric cancer setting, the most common adverse reactions (≥ 10%) that were increased (≥ 5% difference) in the Trastuzumab arm as compared to the chemotherapy alone arm were neutropenia, diarrhea, fatigue, anemia, stomatitis, weight loss, upper respiratory tract infections, fever, thrombocytopenia, mucosal inflammation, nasopharyngitis, and dysgeusia. The most common adverse reactions which resulted in discontinuation of treatment on the Trastuzumab-containing arm in the absence of disease progression were infection, diarrhea, and febrile neutropenia.
- Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
Adjuvant Breast Cancer Studies
- The data below reflect exposure to one-year Trastuzumab therapy across three randomized, open-label studies, Studies 1, 2, and 3, with (n=3678) or without (n= 3363) trastuzumab in the adjuvant treatment of breast cancer.
The data summarized in Table 3 below, from Study 3, reflect exposure to Trastuzumab in 1678 patients; the median treatment duration was 51 weeks and median number of infusions was 18. Among the 3386 patients enrolled in the observation and one-year Trastuzumab arms of Study 3 at a median duration of follow-up of 12.6 months in the Trastuzumab arm, the median age was 49 years (range: 21 to 80 years), 83% of patients were Caucasian, and 13% were Asian.
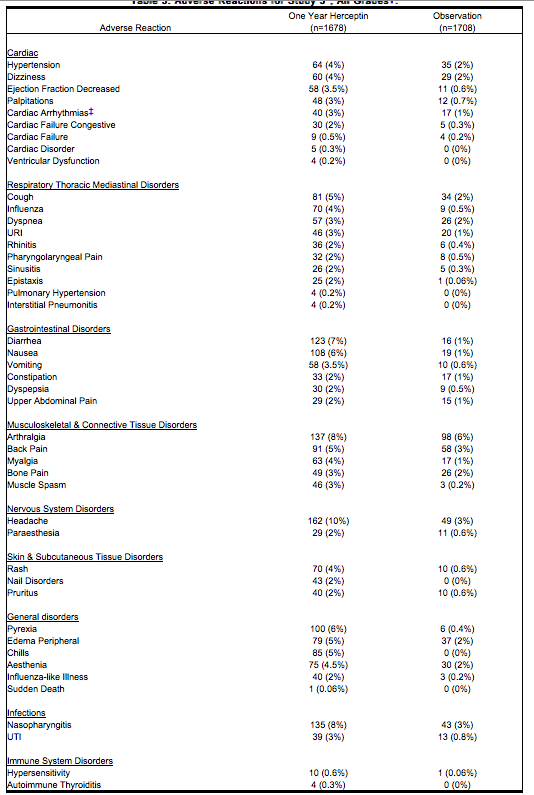
In Study 3, a comparison of 3-weekly Trastuzumab treatment for two years versus one year was also performed. The rate of asymptomatic cardiac dysfunction was increased in the 2-year Trastuzumab treatment arm (8.1% versus 4.6% in the one-year Trastuzumab treatment arm). More patients experienced at least one adverse reaction of grade 3 or higher in the 2-year Trastuzumab treatment arm (20.4%) compared with the one-year Trastuzumab treatment arm (16.3%). The safety data from Studies 1 and 2 were obtained from 3655 patients, of whom 2000 received Trastuzumab; the median treatment duration was 51 weeks. The median age was 49 years (range: 24–80); 84% of patients were White, 7% Black, 4% Hispanic, and 3% Asian. In Study 1, only Grade 3–5 adverse events, treatment-related Grade 2 events, and Grade 2–5 dyspnea were collected during and for up to 3 months following protocol-specified treatment. The following non-cardiac adverse reactions of Grade 2–5 occurred at an incidence of at least 2% greater among patients receiving Trastuzumab plus chemotherapy as compared to chemotherapy alone: fatigue (29.5% vs. 22.4%), infection (24.0% vs. 12.8%), hot flashes (17.1% vs. 15.0%), anemia (12.3% vs. 6.7%), dyspnea (11.8% vs. 4.6%), rash/desquamation (10.9% vs. 7.6%), leukopenia (10.5% vs. 8.4%), neutropenia (6.4% vs. 4.3%), headache (6.2% vs. 3.8%), pain (5.5% vs. 3.0%), edema (4.7% vs. 2.7%) and insomnia (4.3% vs. 1.5%). The majority of these events were Grade 2 in severity. In Study 2, data collection was limited to the following investigator-attributed treatment-related adverse reactions: NCI-CTC Grade 4 and 5 hematologic toxicities, Grade 3–5 non-hematologic toxicities, selected Grade 2–5 toxicities associated with taxanes (myalgia, arthralgias, nail changes, motor neuropathy, sensory neuropathy) and Grade 1–5 cardiac toxicities occurring during chemotherapy and/or Trastuzumab treatment. The following non-cardiac adverse reactions of Grade 2–5 occurred at an incidence of at least 2% greater among patients receiving Trastuzumab plus chemotherapy as compared to chemotherapy alone: arthralgia (12.2% vs. 9.1%), nail changes (11.5% vs.6.8%), dyspnea (2.4% vs. 0.2%), and diarrhea (2.2% vs. 0%). The majority of these events were Grade 2 in severity. Safety data from Study 4 reflect exposure to Trastuzumab as part of an adjuvant treatment regimen from 2124 patients receiving at least one dose of study treatment [AC-TH: n = 1068; TCH: n = 1056]. The overall median treatment duration was 54 weeks in both the AC-TH and TCH arms. The median number of infusions was 26 in the AC-TH arm and 30 in the TCH arm, including weekly infusions during the chemotherapy phase and every three week dosing in the monotherapy period. Among these patients, the median age was 49 years (range 22 to 74 years). In Study 4, the toxicity profile was similar to that reported in Studies 1, 2, and 3 with the exception of a low incidence of CHF in the TCH arm.
Metastatic Breast Cancer Studies
The data below reflect exposure to Trastuzumab in one randomized, open-label study, Study 5, of chemotherapy with (n=235) or without (n=234) trastuzumab in patients with metastatic breast cancer, and one single-arm study (Study 6; n=222) in patients with metastatic breast cancer. Data in Table 4 are based on Studies 5 and 6. Among the 464 patients treated in Study 5, the median age was 52 years (range: 25–77 years). Eighty-nine percent were White, 5% Black, 1% Asian and 5% other racial/ethnic groups. All patients received 4 mg/kg initial dose of Trastuzumab followed by 2 mg/kg weekly. The percentages of patients who received Trastuzumab treatment for ≥ 6 months and ≥ 12 months were 58% and 9%, respectively. Among the 352 patients treated in single agent studies (213 patients from Study 6), the median age was 50 years (range 28–86 years), 86% were White, 3% were Black, 3% were Asian, and 8% in other racial/ethnic groups. Most of the patients received 4 mg/kg initial dose of Trastuzumab followed by 2 mg/kg weekly. The percentages of patients who received Trastuzumab treatment for ≥ 6 months and ≥ 12 months were 31% and 16%, respectively.
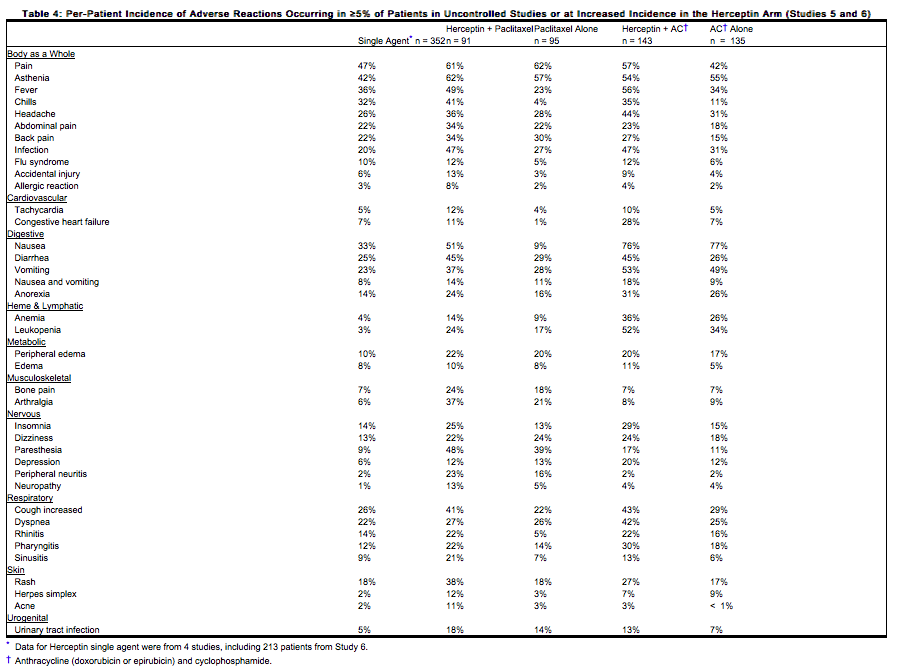
Metastatic Gastric Cancer
The data below are based on the exposure of 294 patients to Trastuzumab in combination with a fluoropyrimidine (capecitabine or 5-FU) and cisplatin (Study 7). In the Trastuzumab plus chemotherapy arm, the initial dose of Trastuzumab 8 mg/kg was administered on Day 1 (prior to chemotherapy) followed by 6 mg/kg every 21 days until disease progression. Cisplatin was administered at 80 mg/m2 on Day 1 and the fluoropyrimidine was administered as either capecitabine 1000 mg/m2 orally twice a day on Days 1-14 or 5-fluorouracil 800 mg/m2/day as a continuous intravenous infusion Days 1 through 5. Chemotherapy was administered for six 21-day cycles. Median duration of Trastuzumab treatment was 21 weeks; median number of Trastuzumab infusions administered was eight.
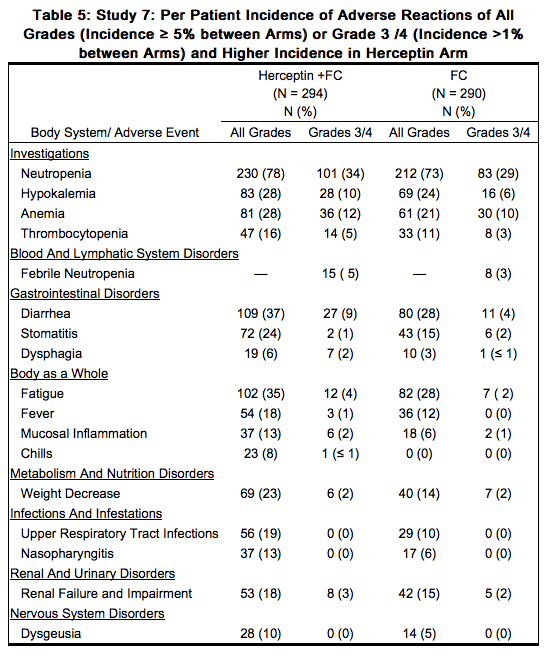
The following subsections provide additional detail regarding adverse reactions observed in clinical trials of adjuvant breast, metastatic breast cancer, metastatic gastric cancer, or post-marketing experience.
Serial measurement of cardiac function (LVEF) was obtained in clinical trials in the adjuvant treatment of breast cancer. In Study 3, the median duration of follow-up was 12.6 months (12.4 months in the observation arm; 12.6 months in the 1-year Trastuzumab arm); and in Studies 1 and 2, 7.9 years in the AC-T arm, 8.3 years in the AC-TH arm. In Studies 1 and 2, 6% of all randomized patients with post-AC LVEF evaluation were not permitted to initiate Trastuzumab following completion of AC chemotherapy due to cardiac dysfunction (LVEF < LLN or ≥ 16 point decline in LVEF from baseline to end of AC). Following initiation of Trastuzumab therapy, the incidence of new-onset dose-limiting myocardial dysfunction was higher among patients receiving Trastuzumab and paclitaxel as compared to those receiving paclitaxel alone in Studies 1 and 2, and in patients receiving one-year Trastuzumab monotherapy compared to observation in Study 3 (see Table 6, Figures 1 and 2). The per-patient incidence of new-onset cardiac dysfunction, as measured by LVEF, remained similar when compared to the analysis performed at a median follow-up of 2.0 years in the AC-TH arm. This analysis also showed evidence of reversibility of left ventricular dysfunction, with 64.5% of patients who experienced symptomatic CHF in the AC-TH group being asymptomatic at latest follow-up, and 90.3% having full or partial LVEF recovery.
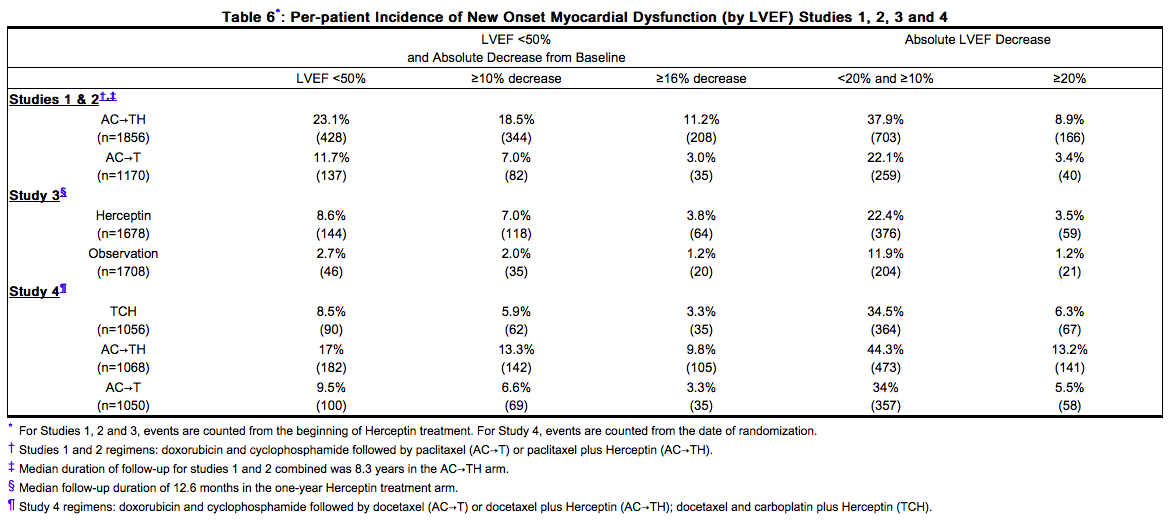
Figure 1
Studies 1 and 2: Cumulative Incidence of Time to First LVEF Decline of ≥ 10 Percentage Points from Baseline and to Below 50% with Death as a Competing Risk Event
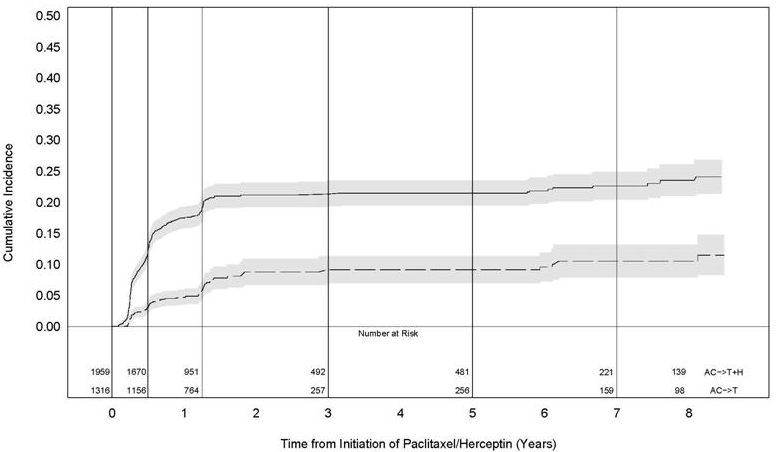
Time 0 is initiation of paclitaxel or Trastuzumab + paclitaxel therapy.
Figure 2
Study 3: Cumulative Incidence of Time to First LVEF Decline of ≥ 10 Percentage Points from Baseline and to Below 50% with Death as a Competing Risk Event
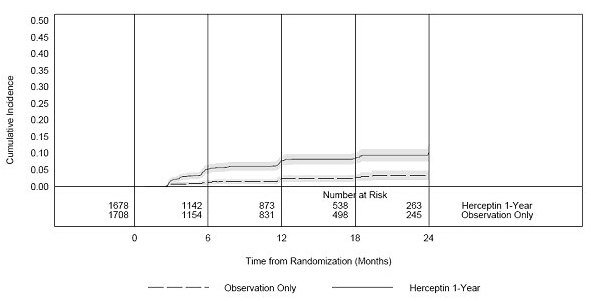
Time 0 is the date of randomization.
Figure 3
Study 4: Cumulative Incidence of Time to First LVEF Decline of ≥10 Percentage Points from Baseline and to Below 50% with Death as a Competing Risk Event
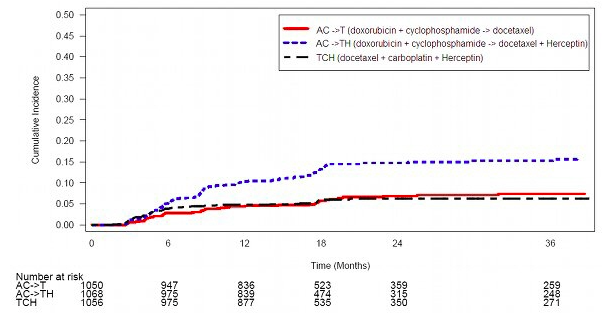
Time 0 is the date of randomization. The incidence of treatment emergent congestive heart failure among patients in the metastatic breast cancer trials was classified for severity using the New York Heart Association classification system (I–IV, where IV is the most severe level of cardiac failure) (see Table 2). In the metastatic breast cancer trials the probability of cardiac dysfunction was highest in patients who received Trastuzumab concurrently with anthracyclines. In Study 7, 5.0% of patients in the Trastuzumab plus chemotherapy arm compared to 1.1% of patients in the chemotherapy alone arm had LVEF value below 50% with a ≥ 10% absolute decrease in LVEF from pretreatment values.
Infusion Reactions
During the first infusion with Trastuzumab, the symptoms most commonly reported were chills and fever, occurring in approximately 40% of patients in clinical trials. Symptoms were treated with acetaminophen, diphenhydramine, and meperidine (with or without reduction in the rate of Trastuzumab infusion); permanent discontinuation of Trastuzumab for infusional toxicity was required in < 1% of patients. Other signs and/or symptoms may include nausea, vomiting, pain (in some cases at tumor sites), rigors, headache, dizziness, dyspnea, hypotension, elevated blood pressure, rash, and asthenia. Infusional toxicity occurred in 21% and 35% of patients, and was severe in 1.4% and 9% of patients, on second or subsequent Trastuzumab infusions administered as monotherapy or in combination with chemotherapy, respectively. In the post-marketing setting, severe infusion reactions, including hypersensitivity, anaphylaxis, and angioedema have been reported.
In randomized controlled clinical trials, the overall incidence of anemia (30% vs. 21% [Study 5]), of selected NCI-CTC Grade 2–5 anemia (12.3% vs. 6.7% [Study 1]), and of anemia requiring transfusions (0.1% vs. 0 patients [Study 2]) were increased in patients receiving Trastuzumab and chemotherapy compared with those receiving chemotherapy alone. Following the administration of Trastuzumab as a single agent (Study 6), the incidence of NCI-CTC Grade 3 anemia was < 1%. In Study 7 (metastatic gastric cancer) on the Trastuzumab containing arm as compared to the chemotherapy alone arm the overall incidence of anemia was 28% compared 21% and of NCI CTC Grade 3/4 anemia was 12.2% compared to 10.3%.
In randomized controlled clinical trials in the adjuvant setting, the incidence of selected NCI-CTC Grade 4–5 neutropenia (1.7% vs. 0.8% [Study 2]) and of selected Grade 2–5 neutropenia (6.4% vs. 4.3% [Study 1]) were increased in patients receiving Trastuzumab and chemotherapy compared with those receiving chemotherapy alone. In a randomized, controlled trial in patients with metastatic breast cancer, the incidences of NCI-CTC Grade 3/4 neutropenia (32% vs. 22%) and of febrile neutropenia (23% vs. 17%) were also increased in patients randomized to Trastuzumab in combination with myelosuppressive chemotherapy as compared to chemotherapy alone. In Study 7 (metastatic gastric cancer) on the Trastuzumab containing arm as compared to the chemotherapy alone arm, the incidence of NCI CTC Grade 3/4 neutropenia was 36.8% compared to 28.9%; febrile neutropenia 5.1% compared to 2.8%.
Infection
The overall incidences of infection (46% vs. 30% [Study 5]), of selected NCI-CTC Grade 2–5 infection/febrile neutropenia (24.3% vs. 13.4% [Study 1]) and of selected Grade 3–5 infection/febrile neutropenia (2.9% vs. 1.4%) [Study 2]), were higher in patients receiving Trastuzumab and chemotherapy compared with those receiving chemotherapy alone. The most common site of infections in the adjuvant setting involved the upper respiratory tract, skin, and urinary tract. In Study 4, the overall incidence of infection was higher with the addition of Trastuzumab to AC-T but not to TCH [44% (AC-TH), 37% (TCH), 38% (AC-T)]. The incidences of NCI-CTC Grade 3-4 infection were similar [25% (AC-TH), 21% (TCH), 23% (AC-T)] across the three arms. In a randomized, controlled trial in treatment of metastatic breast cancer, the reported incidence of febrile neutropenia was higher (23% vs. 17%) in patients receiving Trastuzumab in combination with myelosuppressive chemotherapy as compared to chemotherapy alone.
Pulmonary Toxicity
Adjuvant Breast Cancer
Among women receiving adjuvant therapy for breast cancer, the incidence of selected NCI-CTC Grade 2–5 pulmonary toxicity (14.3% vs. 5.4% [Study 1]) and of selected NCI-CTC Grade 3–5 pulmonary toxicity and spontaneous reported Grade 2 dyspnea (3.4% vs. 0.9% [Study 2]) was higher in patients receiving Trastuzumab and chemotherapy compared with chemotherapy alone. The most common pulmonary toxicity was dyspnea (NCI-CTC Grade 2–5: 11.8% vs. 4.6% [Study 1]; NCI-CTC Grade 2–5: 2.4% vs. 0.2% [Study 2]). Pneumonitis/pulmonary infiltrates occurred in 0.7% of patients receiving Trastuzumab compared with 0.3% of those receiving chemotherapy alone. Fatal respiratory failure occurred in 3 patients receiving Trastuzumab, one as a component of multi-organ system failure, as compared to 1 patient receiving chemotherapy alone. In Study 3, there were 4 cases of interstitial pneumonitis in the one-year Trastuzumab treatment arm compared to none in the observation arm at a median follow-up duration of 12.6 months.
Metastatic Breast Cancer
Among women receiving Trastuzumab for treatment of metastatic breast cancer, the incidence of pulmonary toxicity was also increased. Pulmonary adverse events have been reported in the post-marketing experience as part of the symptom complex of infusion reactions. Pulmonary events include bronchospasm, hypoxia, dyspnea, pulmonary infiltrates, pleural effusions, non-cardiogenic pulmonary edema, and acute respiratory distress syndrome. Thrombosis/Embolism In 4 randomized, controlled clinical trials, the incidence of thrombotic adverse events was higher in patients receiving Trastuzumab and chemotherapy compared to chemotherapy alone in three studies (2.6% vs. 1.5% [Study 1], 2.5% and 3.7% vs. 2.2% [Study 4] and 2.1% vs. 0% [Study 5]).
Among women receiving adjuvant therapy for breast cancer, the incidence of NCI-CTC Grade 2–5 diarrhea (6.7% vs. 5.4% [Study 1]) and of NCI-CTC Grade 3–5 diarrhea (2.2% vs. 0% [Study 2]), and of Grade 1–4 diarrhea (7% vs. 1% [Study 3; one-year Trastuzumab treatment at 12.6 months median duration of follow-up]) were higher in patients receiving Trastuzumab as compared to controls. In Study 4, the incidence of Grade 3–4 diarrhea was higher [5.7% AC-TH, 5.5% TCH vs. 3.0% AC-T] and of Grade 1–4 was higher [51% AC-TH, 63% TCH vs. 43% AC-T] among women receiving Trastuzumab. Of patients receiving Trastuzumab as a single agent for the treatment of metastatic breast cancer, 25% experienced diarrhea. An increased incidence of diarrhea was observed in patients receiving Trastuzumab in combination with chemotherapy for treatment of metastatic breast cancer.
Renal Toxicity
In Study 7 (metastatic gastric cancer) on the Trastuzumab-containing arm as compared to the chemotherapy alone arm the incidence of renal impairment was 18% compared to 14.5%. Severe (Grade 3/4) renal failure was 2.7% on the Trastuzumab-containing arm compared to 1.7% on the chemotherapy only arm. Treatment discontinuation for renal insufficiency/failure was 2% on the Trastuzumab-containing arm and 0.3% on the chemotherapy only arm. In the postmarketing setting, rare cases of nephrotic syndrome with pathologic evidence of glomerulopathy have been reported. The time to onset ranged from 4 months to approximately 18 months from initiation of Trastuzumab therapy. Pathologic findings included membranous glomerulonephritis, focal glomerulosclerosis, and fibrillary glomerulonephritis. Complications included volume overload and congestive heart failure.
Immunogenicity
As with all therapeutic proteins, there is a potential for immunogenicity. Among 903 women with metastatic breast cancer, human anti-human antibody (HAHA) to Trastuzumab was detected in one patient using an enzyme-linked immunosorbent assay (ELISA). This patient did not experience an allergic reaction. Samples for assessment of HAHA were not collected in studies of adjuvant breast cancer. The incidence of antibody formation is highly dependent on the sensitivity and the specificity of the assay. Additionally, the observed incidence of antibody (including neutralizing antibody) positivity in an assay may be influenced by several factors including assay methodology, sample handling, timing of sample collection, concomitant medications, and underlying disease. For these reasons, comparison of the incidence of antibodies to Trastuzumab with the incidence of antibodies to other products may be misleading.
Postmarketing Experience
The following adverse reactions have been identified during post approval use of Trastuzumab. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
- Infusion reaction
- Oligohydramnios or oligohydramnios sequence, including pulmonary hypoplasia, skeletal abnormalities, and neonatal death
- Glomerulopathy
Drug Interactions
In Study 5, the mean serum trough concentration of trastuzumab was consistently elevated approximately 1.5–fold, when administered in combination with paclitaxel as compared to trough concentrations of trastuzumab when administered in combination with an anthracycline and cyclophosphamide. In other pharmacokinetic studies, where Trastuzumab was administered in combination with paclitaxel, docetaxel, carboplatin, or doxorubicin, Trastuzumab did not alter the plasma concentrations of these chemotherapeutic agents, or the metabolites that were analyzed. In a drug interaction substudy conducted in patients in Study 7, the pharmacokinetics of cisplatin, capecitabine and their metabolites were not altered when administered in combination with Trastuzumab.
Use in Specific Populations
Pregnancy
Pregnancy Category (FDA): D
Trastuzumab can cause fetal harm when administered to a pregnant woman. In post-marketing reports use of Trastuzumab during pregnancy resulted in cases of oligohydramnios and of oligohydramnios sequence, manifesting as pulmonary hypoplasia, skeletal abnormalities, and neonatal death.
These case reports described oligohydramnios in pregnant women who received Trastuzumab either alone or in combination with chemotherapy. In some case reports, amniotic fluid index increased after Trastuzumab was stopped. In one case, Trastuzumab therapy resumed after the amniotic fluid index improved, and oligohydramnios recurred.
Monitor women exposed to Trastuzumab during pregnancy for oligohydramnios. If oligohydramnios occurs, perform fetal testing that is appropriate for gestational age and consistent with community standards of care. The efficacy of IV hydration in management of oligohydramnios due to Trastuzumab exposure is not known.
Advise women of the potential hazard to the fetus resulting from Trastuzumab exposure during pregnancy. Encourage pregnant women with breast cancer who are using Trastuzumab to enroll in MotHER the Trastuzumab Pregnancy Registry: phone 1 800 690 6720.
No teratogenic effects were observed in offspring from reproduction studies in cynomolgus monkeys at doses up to 25 times the recommended weekly human dose of 2 mg/kg trastuzumab. In mutant mice lacking HER2, embryos died in early gestation. Trastuzumab exposure was reported at delivery in offspring of cynomolgus monkeys treated during the early (Days 20–50 of gestation) or late (Days 120–150 of gestation) fetal development periods, at levels of 15 to 28% of the maternal blood levels.
Pregnancy Category (AUS):
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Trastuzumab in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Trastuzumab during labor and delivery.
Nursing Mothers
It is not known whether Trastuzumab is excreted in human milk, but human IgG is excreted in human milk. Published data suggest that breast milk antibodies do not enter the neonatal and infant circulation in substantial amounts. Trastuzumab was present in the breast milk of lactating cynomolgus monkeys given 12.5 times the recommended weekly human dose of 2 mg/kg of Trastuzumab. Infant monkeys with detectable serum levels of trastuzumab did not have any adverse effects on growth or development from birth to 3 months of age; however, trastuzumab levels in animal breast milk may not accurately reflect human breast milk levels. Because many drugs are secreted in human milk and because of the potential for serious adverse reactions in nursing infants from Trastuzumab, a decision should be made whether to discontinue nursing, or discontinue drug, taking into account the elimination half-life of trastuzumab and the importance of the drug to the mother.
Pediatric Use
The safety and effectiveness of Trastuzumab in pediatric patients has not been established.
Geriatic Use
Trastuzumab has been administered to 386 patients who were 65 years of age or over (253 in the adjuvant treatment and 133 in metastatic breast cancer treatment settings). The risk of cardiac dysfunction was increased in geriatric patients as compared to younger patients in both those receiving treatment for metastatic disease in Studies 5 and 6, or adjuvant therapy in Studies 1 and 2. Limitations in data collection and differences in study design of the 4 studies of Trastuzumab in adjuvant treatment of breast cancer preclude a determination of whether the toxicity profile of Trastuzumab in older patients is different from younger patients. The reported clinical experience is not adequate to determine whether the efficacy improvements (ORR, TTP, OS, DFS) of Trastuzumab treatment in older patients is different from that observed in patients <65 years of age for metastatic disease and adjuvant treatment. In Study 7 (metastatic gastric cancer), of the 294 patients treated with Trastuzumab 108 (37%) were 65 years of age or older, while 13 (4.4%) were 75 and over. No overall differences in safety or effectiveness were observed.
Gender
There is no FDA guidance on the use of Trastuzumab with respect to specific gender populations.
Race
There is no FDA guidance on the use of Trastuzumab with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Trastuzumab in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Trastuzumab in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Trastuzumab in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Trastuzumab in patients who are immunocompromised.
Administration and Monitoring
Administration
To prevent medication errors, it is important to check the vial labels to ensure that the drug being prepared and administered is Trastuzumab and not ado-trastuzumab emtansine.
Reconstitution
Reconstitute each 440 mg vial of Trastuzumab with 20 mL of Bacteriostatic Water for Injection (BWFI), USP, containing 1.1% benzyl alcohol as a preservative to yield a multi-dose solution containing 21 mg/mL trastuzumab. In patients with known hypersensitivity to benzyl alcohol, reconstitute with 20 mL of Sterile Water for Injection (SWFI) without preservative to yield a single use solution. Use appropriate aseptic technique when performing the following reconstitution steps:
- Using a sterile syringe, slowly inject the 20 mL of diluent into the vial containing the lyophilized cake of Trastuzumab. The stream of diluent should be directed into the lyophilized cake.
- Swirl the vial gently to aid reconstitution. DO NOT SHAKE.
- Slight foaming of the product may be present upon reconstitution. Allow the vial to stand undisturbed for approximately 5 minutes.
- Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit. Inspect visually for particulates and discoloration. The solution should be free of visible particulates, clear to slightly opalescent and colorless to pale yellow.
- Store reconstituted Trastuzumab at 2–8° C; discard unused Trastuzumab after 28 days. If Trastuzumab is reconstituted with SWFI without preservative, use immediately and discard any unused portion.
Dilution
- Determine the dose (mg) of Trastuzumab. Calculate the volume of the 21 mg/mL reconstituted Trastuzumab solution needed, withdraw this amount from the vial and add it to an infusion bag containing 250 mL of 0.9% Sodium Chloride Injection, USP.
DO NOT USE DEXTROSE (5%) SOLUTION.
- Gently invert the bag to mix the solution.
Monitoring
FDA Package Insert for Trastuzumab contains no information regarding drug monitoring.
IV Compatibility
There is limited information about the IV Compatibility.
Overdosage
There is no experience with overdosage in human clinical trials. Single doses higher than 8 mg/kg have not been tested.
Pharmacology
Trastuzumab?
| |
| Therapeutic monoclonal antibody | |
| Source | zu/o |
| Target | HER2/neu |
| Identifiers | |
| CAS number | |
| ATC code | L01 |
| PubChem | ? |
| DrugBank | |
| Chemical data | |
| Formula | Template:OrganicBox atomTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox |
| Mol. mass | 145531.5 g/mol |
| Pharmacokinetic data | |
| Bioavailability | ? |
| Metabolism | Unknown, possibly reticuloendothelial system. |
| Half life | 2-12 days |
| Excretion | ? |
| Therapeutic considerations | |
| Pregnancy cat. |
?(US) X |
| Legal status | |
| Routes | Intravenous |
Mechanism of Action
The HER2 (or c-erbB2) proto-oncogene encodes a transmembrane receptor protein of 185 kDa, which is structurally related to the epidermal growth factor receptor. Trastuzumab has been shown, in both in vitro assays and in animals, to inhibit the proliferation of human tumor cells that overexpress HER2. Trastuzumab is a mediator of antibody-dependent cellular cytotoxicity (ADCC). In vitro, Trastuzumab-mediated ADCC has been shown to be preferentially exerted on HER2 overexpressing cancer cells compared with cancer cells that do not overexpress HER2.
Structure
Trastuzumab is a humanized IgG1 kappa monoclonal antibody that selectively binds with high affinity to the extracellular domain of the human epidermal growth factor receptor 2 protein, HER2. Trastuzumab is produced by recombinant DNA technology in a mammalian cell (Chinese Hamster Ovary) culture containing the antibiotic gentamicin. Gentamicin is not detectable in the final product. Trastuzumab is a sterile, white to pale yellow, preservative-free lyophilized powder for intravenous administration. Each multi-use vial of Trastuzumab contains 440 mg trastuzumab, 400 mg α,α-trehalose dihydrate, 9.9 mg L-histidine HCl, 6.4 mg L-histidine, and 1.8 mg polysorbate 20, USP. Reconstitution with 20 mL of the appropriate diluent (BWFI or SWFI) yields a solution containing 21 mg/mL trastuzumab, at a pH of approximately 6.
Pharmacodynamics
FDA Package Insert for Trastuzumab contains no information regarding Pharmacokinetics.
Pharmacokinetics
The pharmacokinetics of trastuzumab were studied in women with metastatic breast cancer. Short duration intravenous infusions of 10 to 500 mg Trastuzumab once weekly demonstrated dose-dependent pharmacokinetics. Mean half-life increased and clearance decreased with increasing dose level. The half-life averaged 2 and 12 days at the 10 and 500 mg dose levels, respectively. The volume of distribution of trastuzumab was approximately that of serum volume (44 mL/kg). At the highest weekly dose studied (500 mg), mean peak serum concentrations were 377 mcg/mL. In studies using an initial dose of 4 mg/kg followed by a weekly dose of 2 mg/kg, a mean half-life of 6 days (range 1–32 days) was observed. Between weeks 16 and 32, trastuzumab serum concentrations reached a steady state with mean trough and peak concentrations of approximately 79 mcg/mL and 123 mcg/mL, respectively. In a study of women receiving adjuvant therapy for breast cancer, a mean half-life of trastuzumab of 16 days (range: 11–23 days) was observed after an initial dose of 8 mg/kg followed by a dose of 6 mg/kg every three weeks. Between weeks 6 and 37, trastuzumab serum concentrations reached a steady-state with mean trough and peak concentrations of 63 mcg/mL and 216 mcg/mL, respectively. In patients with metastatic gastric cancer (Study 7), mean serum trastuzumab trough concentrations at steady state were 24 to 63% lower as compared to the concentrations observed in patients with breast cancer receiving treatment for metastatic disease in combination with paclitaxel, as monotherapy for metastatic disease, or as adjuvant monotherapy. Sixty-four percent (286/447) of women with metastatic breast cancer had detectable circulating extracellular domain of the HER2 receptor (shed antigen), which ranged as high as 1880 ng/mL (median 11 ng/mL). Patients with higher baseline shed antigen levels were more likely to have lower serum trough concentrations. Data suggest that the disposition of trastuzumab is not altered based on age or serum creatinine (≤2.0 mg creatinine/dL).
Nonclinical Toxicology
Carcinogenesis, Mutagenesis, Impairment of Fertility
Trastuzumab has not been tested for carcinogenic potential. No evidence of mutagenic activity was observed when trastuzumab was tested in the standard Ames bacterial and human peripheral blood lymphocyte mutagenicity assays, at concentrations of up to 5000 mcg/mL. In an in vivo micronucleus assay, no evidence of chromosomal damage to mouse bone marrow cells was observed following bolus intravenous doses of up to 118 mg/kg Trastuzumab. A fertility study conducted in female cynomolgus monkeys at doses up to 25 times the weekly recommended human dose of 2 mg/kg trastuzumab and has revealed no evidence of impaired fertility, as measured by menstrual cycle duration and female sex hormone levels. Studies to evaluate the effects of trastuzumab on male fertility have not been conducted.
Animal Toxicology and/or Pharmacology
Reproductive Toxicology Studies
Reproductive toxicology studies have been conducted in cynomolgus monkeys at doses up to 25 times the weekly recommended human dose of 2 mg/kg Trastuzumab and have revealed no evidence of impaired fertility or harm to the fetus. However, HER2 protein expression is high in many embryonic tissues including cardiac and neural tissues; in mutant mice lacking HER2, embryos died in early gestation. Placental transfer of trastuzumab was detected at Caesarean section in offspring from pregnant cynomolgus monkeys dosed during the early (Days 20–50 of gestation) or late (Days 120–150 of gestation) fetal development periods.
Clinical Studies
Adjuvant Breast Cancer
The safety and efficacy of Trastuzumab in women receiving adjuvant chemotherapy for HER2 overexpressing breast cancer, were evaluated in an integrated analysis of two randomized, open-label, clinical trials (Studies 1 and 2) with a total of 4063 women at the protocol-specified final overall survival analysis, a third randomized, open-label, clinical trial (Study 3) with a total of 3386 women at definitive Disease Free Survival analysis for one-year Trastuzumab treatment versus observation, and a fourth randomized, open-label clinical trial with a total of 3222 patients (Study 4).
Studies 1 and 2
In Studies 1 and 2, breast tumor specimens were required to show HER2 overexpression (3+ by IHC) or gene amplification (by FISH). HER2 testing was verified by a central laboratory prior to randomization (Study 2) or was required to be performed at a reference laboratory (Study 1). Patients with a history of active cardiac disease based on symptoms, abnormal electrocardiographic, radiologic, or left ventricular ejection fraction findings or uncontrolled hypertension (diastolic > 100 mmHg or systolic > 200 mmHg) were not eligible. Patients were randomized (1:1) to receive doxorubicin and cyclophosphamide followed by paclitaxel (AC→paclitaxel) alone or paclitaxel plus Trastuzumab (AC→paclitaxel + Trastuzumab). In both trials, patients received four 21-day cycles of doxorubicin 60 mg/m2 and cyclophosphamide 600 mg/m2. Paclitaxel was administered either weekly (80 mg/m2) or every 3 weeks (175 mg/m2) for a total of 12 weeks in Study 1; paclitaxel was administered only by the weekly schedule in Study 2. Trastuzumab was administered at 4 mg/kg on the day of initiation of paclitaxel and then at a dose of 2 mg/kg weekly for a total of 52 weeks. Trastuzumab treatment was permanently discontinued in patients who developed congestive heart failure, or persistent/recurrent LVEF decline. Radiation therapy, if administered, was initiated after the completion of chemotherapy. Patients with ER+ and/or PR+ tumors received hormonal therapy. The primary endpoint of the combined efficacy analysis was Disease-free survival (DFS), defined as the time from randomization to recurrence, occurrence of contralateral breast cancer, other second primary cancer, or death. The secondary endpoint was overall survival (OS). A total of 3752 patients were included in the joint efficacy analysis of the primary endpoint of DFS following a median follow-up of 2.0 years in the AC→paclitaxel + Trastuzumab arm. The pre-planned final OS analysis from the joint analysis included 4063 patients and was performed when 707 deaths had occurred after a median follow-up of 8.3 years in the AC→paclitaxel + Trastuzumab arm. The data from both arms in Study 1 and two of the three study arms in Study 2 were pooled for efficacy analyses. The patients included in the primary DFS analysis had a median age of 49 years (range, 22–80 years; 6% > 65 years), 84% were white, 7% black, 4% Hispanic, and 4% Asian/Pacific Islander. Disease characteristics included 90% infiltrating ductal histology, 38% T1, 91% nodal involvement, 27% intermediate and 66% high grade pathology, and 53% ER+ and/or PR+ tumors. Similar demographic and baseline characteristics were reported for the efficacy evaluable population, after 8.3 years of median follow-up in the AC→paclitaxel + Trastuzumab arm.
Study 3
In Study 3, breast tumor specimens were required to show HER2 overexpression (3+ by IHC) or gene amplification (by FISH) as determined at a central laboratory. Patients with node-negative disease were required to have ≥ T1c primary tumor. Patients with a history of congestive heart failure or LVEF <55%, uncontrolled arrhythmias, angina requiring medication, clinically significant valvular heart disease, evidence of transmural infarction on ECG, poorly controlled hypertension (systolic > 180 mm Hg or diastolic > 100 mm Hg) were not eligible. Study 3 was designed to compare one and two years of three-weekly Trastuzumab treatment versus observation in patients with HER2 positive EBC following surgery, established chemotherapy and radiotherapy (if applicable). Patients were randomized (1:1:1) upon completion of definitive surgery, and at least four cycles of chemotherapy to receive no additional treatment, or one year of Trastuzumab treatment or two years of Trastuzumab treatment. Patients undergoing a lumpectomy had also completed standard radiotherapy. Patients with ER+ and/or PgR+ disease received systemic adjuvant hormonal therapy at investigator discretion. Trastuzumab was administered with an initial dose of 8 mg/kg followed by subsequent doses of 6 mg/kg once every three weeks. The main outcome measure was disease-free survival (DFS), defined as in Studies 1 and 2. A protocol specified interim efficacy analysis comparing one-year Trastuzumab treatment to observation was performed at a median follow-up duration of 12.6 months in the Trastuzumab arm and formed the basis for the definitive DFS results from this study. Among the 3386 patients randomized to the observation (n =1693) and Trastuzumab one-year (n= 1693) treatment arms, the median age was 49 years (range 21–80), 83% were Caucasian, and 13% were Asian. Disease characteristics: 94% infiltrating ductal carcinoma, 50% ER+ and/or PgR+, 57% node positive, 32% node negative, and in 11% of patients, nodal status was not assessable due to prior neo-adjuvant chemotherapy. Ninety-six percent (1055/1098) of patients with node-negative disease had high risk features: among the 1098 patients with node-negative disease, 49% (543) were ER− and PgR−, and 47% (512) were ER and/or PgR + and had at least one of the following high risk features: pathological tumor size greater than 2 cm, Grade 2–3, or age < 35 years. Prior to randomization, 94% of patients had received anthracycline-based chemotherapy regimens. After the definitive DFS results comparing observation to one-year Trastuzumab treatment were disclosed, a prospectively planned analysis that included comparison of one year versus two years of Trastuzumab treatment at a median follow-up duration of 8 years was performed. Based on this analysis, extending Trastuzumab treatment for a duration of two years did not show additional benefit over treatment for one year [Hazard Ratios of two-years Trastuzumab versus one-year Trastuzumab treatment in the intent to treat (ITT) population for Disease Free Survival (DFS) = 0.99 (95% CI: 0.87, 1.13), p-value = 0.90 and Overall Survival (OS) = 0.98 (0.83, 1.15); p-value= 0.78].
Study 4
In Study 4, breast tumor specimens were required to show HER2 gene amplification (FISH+ only) as determined at a central laboratory. Patients were required to have either node-positive disease, or node-negative disease with at least one of the following high-risk features: ER/PR-negative, tumor size > 2 cm, age < 35 years, or histologic and/or nuclear Grade 2 or 3. Patients with a history of CHF, myocardial infarction, Grade 3 or 4 cardiac arrhythmia, angina requiring medication, clinically significant valvular heart disease, poorly controlled hypertension (diastolic > 100 mmHg), any T4 or N2 or known N3 or M1 breast cancer were not eligible. Patients were randomized (1:1:1) to receive doxorubicin and cyclophosphamide followed by docetaxel (AC-T), doxorubicin and cyclophosphamide followed by docetaxel plus Trastuzumab (AC-TH), or docetaxel and carboplatin plus Trastuzumab (TCH). In both the AC-T and AC-TH arms, doxorubicin 60 mg/m2 and cyclophosphamide 600 mg/m2 were administered every 3 weeks for four cycles; docetaxel 100 mg/m 2 was administered every 3 weeks for four cycles. In the TCH arm, docetaxel 75 mg/m2 and carboplatin (at a target AUC of 6 mg/mL/min as a 30- to 60-minute infusion) were administered every 3 weeks for six cycles. Trastuzumab was administered weekly (initial dose of 4 mg/kg followed by weekly dose of 2 mg/kg) concurrently with either T or TC, and then every 3 weeks (6 mg/kg) as monotherapy for a total of 52 weeks. Radiation therapy, if administered, was initiated after completion of chemotherapy. Patients with ER+ and/or PR+ tumors received hormonal therapy. Disease-free survival (DFS) was the main outcome measure. Among the 3222 patients randomized, the median age was 49 (range 22 to 74 years; 6% ≥ 65 years). Disease characteristics included 54% ER+ and/or PR+ and 71% node positive. Prior to randomization, all patients underwent primary surgery for breast cancer. The results for DFS for the integrated analysis of Studies 1 and 2, Study 3, and Study 4 and OS results for the integrated analysis of Studies 1 and 2, and Study 3 are presented in Table 7. For Studies 1 and 2, the duration of DFS following a median follow-up of 2.0 years in the AC→TH arm, is presented in Figure 4, and the duration of OS after a median follow-up of 8.3 years in the AC→TH arm is presented in Figure 5. The duration of DFS for Study 4 is presented in Figure 6. Across all four studies, at the time of definitive DFS analysis, there were insufficient numbers of patients within each of the following subgroups to determine if the treatment effect was different from that of the overall patient population: patients with low tumor grade, patients within specific ethnic/racial subgroups (Black, Hispanic, Asian/Pacific Islander patients), and patients > 65 years of age. For Studies 1 and 2, the OS hazard ratio was 0.64 (95% CI: 0.55, 0.74). At 8.3 years of median follow up [AC→TH], the survival rate was estimated to be 86.9% in the AC→TH arm and 79.4% in the AC→T arm. The final OS analysis results from Studies 1 and 2 indicate that OS benefit by age, hormone receptor status, number of positive lymph nodes, tumor size and grade, and surgery/radiation therapy, was consistent with the treatment effect in the overall population. In patients ≤ 50 years of age (n=2197), the OS hazard ratio was 0.65 (95% CI: 0.52, 0.81) and in patients > 50 years of age (n=1866), the OS hazard ratio was 0.63 (95% CI: 0.51, 0.78). In the subgroup of patients with hormone receptor-positive disease (ER-positive and/or PR-positive) (n=2223), the hazard ratio for OS was 0.63 (95% CI: 0.51, 0.78). In the subgroup of patients with hormone receptor-negative disease (ER-negative and PR-negative) (n=1830), the hazard ratio for OS was 0.64 (95% CI: 0.52, 0.80). In the subgroup of patients with tumor size ≤2 cm (n=1604), the hazard ratio for OS was 0.52 (95% CI: 0.39, 0.71). In the subgroup of patients with tumor size >2 cm (n=2448), the hazard ratio for OS was 0.67 (95% CI: 0.56, 0.80).
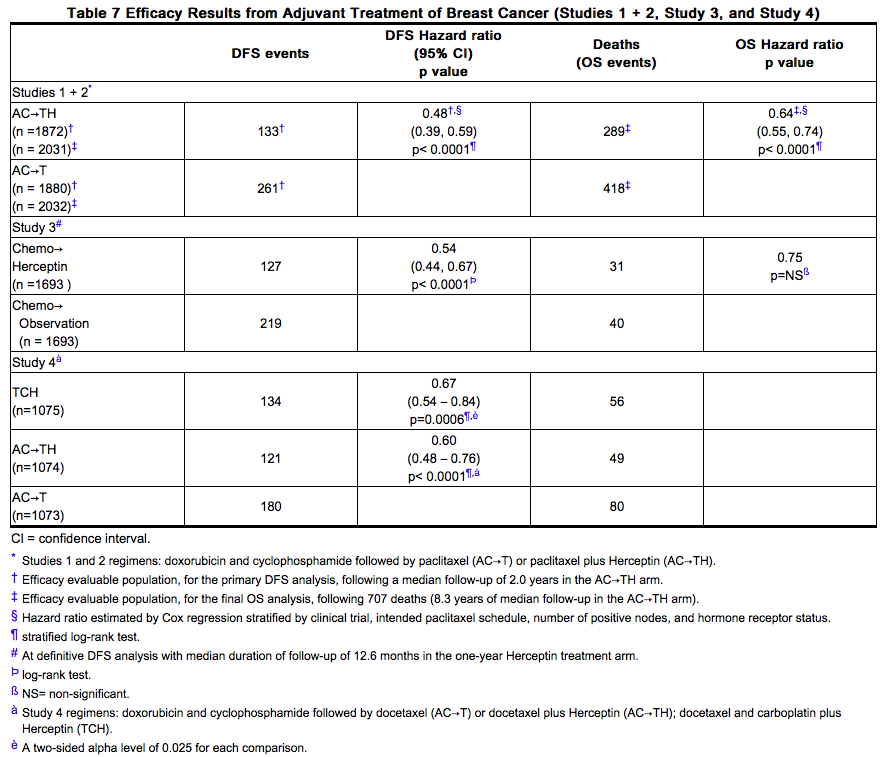
Figure 4
Duration of Disease-Free Survival in Patients with Adjuvant Treatment of Breast Cancer (Studies 1 and 2)
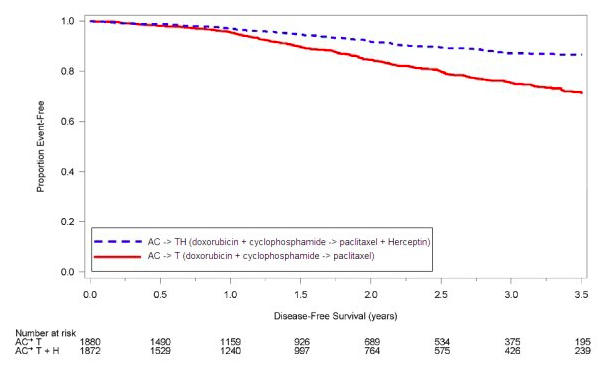

Exploratory analyses of DFS as a function of HER2 overexpression or gene amplification were conducted for patients in Studies 2 and 3, where central laboratory testing data were available. The results are shown in Table 8. The number of events in Study 2 was small with the exception of the IHC 3+/FISH+ subgroup, which constituted 81% of those with data. Definitive conclusions cannot be drawn regarding efficacy within other subgroups due to the small number of events. The number of events in Study 3 was adequate to demonstrate significant effects on DFS in the IHC 3+/FISH unknown and the FISH +/IHC unknown subgroups.
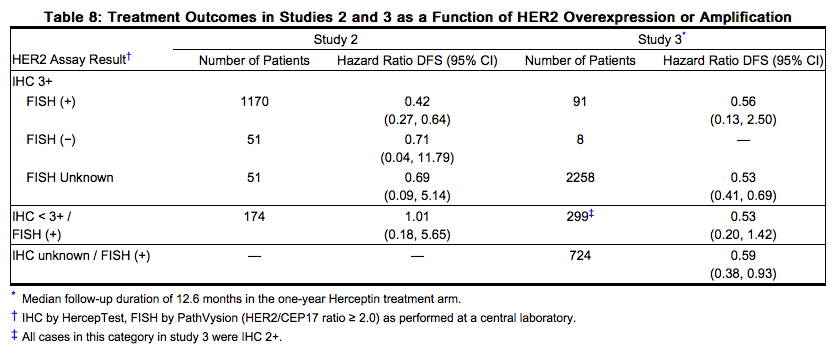
Metastatic Breast Cancer
The safety and efficacy of Trastuzumab in treatment of women with metastatic breast cancer were studied in a randomized, controlled clinical trial in combination with chemotherapy (Study 5, n=469 patients) and an open-label single agent clinical trial (Study 6, n=222 patients). Both trials studied patients with metastatic breast cancer whose tumors overexpress the HER2 protein. Patients were eligible if they had 2 or 3 levels of overexpression (based on a 0 to 3 scale) by immunohistochemical assessment of tumor tissue performed by a central testing lab. Previously Untreated Metastatic Breast Cancer (Study 5) Study 5 was a multicenter, randomized, open-label clinical trial conducted in 469 women with metastatic breast cancer who had not been previously treated with chemotherapy for metastatic disease. Tumor specimens were tested by IHC (Clinical Trial Assay, CTA) and scored as 0, 1+, 2+, or 3+, with 3+ indicating the strongest positivity. Only patients with 2+ or 3+ positive tumors were eligible (about 33% of those screened). Patients were randomized to receive chemotherapy alone or in combination with Trastuzumab given intravenously as a 4 mg/kg loading dose followed by weekly doses of Trastuzumab at 2 mg/kg. For those who had received prior anthracycline therapy in the adjuvant setting, chemotherapy consisted of paclitaxel (175 mg/m2 over 3 hours every 21 days for at least six cycles); for all other patients, chemotherapy consisted of anthracycline plus cyclophosphamide (AC: doxorubicin 60 mg/m2 or epirubicin 75 mg/m2 plus 600 mg/m2 cyclophosphamide every 21 days for six cycles). Sixty-five percent of patients randomized to receive chemotherapy alone in this study received Trastuzumab at the time of disease progression as part of a separate extension study. Based upon the determination by an independent response evaluation committee the patients randomized to Trastuzumab and chemotherapy experienced a significantly longer median time to disease progression, a higher overall response rate (ORR), and a longer median duration of response, as compared with patients randomized to chemotherapy alone. Patients randomized to Trastuzumab and chemotherapy also had a longer median survival (see Table 9). These treatment effects were observed both in patients who received Trastuzumab plus paclitaxel and in those who received Trastuzumab plus AC; however the magnitude of the effects was greater in the paclitaxel subgroup.

Data from Study 5 suggest that the beneficial treatment effects were largely limited to patients with the highest level of HER2 protein overexpression (3+) .
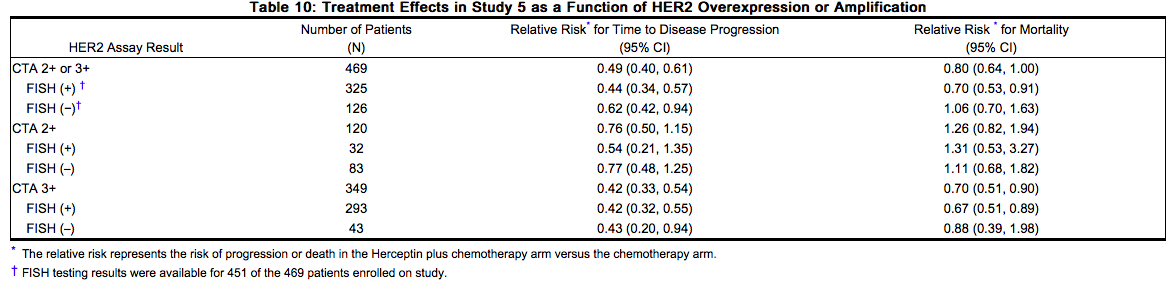
Previously Treated Metastatic Breast Cancer (Study 6)
Trastuzumab was studied as a single agent in a multicenter, open-label, single-arm clinical trial (Study 6) in patients with HER2 overexpressing metastatic breast cancer who had relapsed following one or two prior chemotherapy regimens for metastatic disease. Of 222 patients enrolled, 66% had received prior adjuvant chemotherapy, 68% had received two prior chemotherapy regimens for metastatic disease, and 25% had received prior myeloablative treatment with hematopoietic rescue. Patients were treated with a loading dose of 4 mg/kg IV followed by weekly doses of Trastuzumab at 2 mg/kg IV. The ORR (complete response+partial response), as determined by an independent Response Evaluation Committee, was 14%, with a 2% complete response rate and a 12% partial response rate. Complete responses were observed only in patients with disease limited to skin and lymph nodes. The overall response rate in patients whose tumors tested as CTA 3+ was 18% while in those that tested as CTA 2+, it was 6%.
Metastatic Gastric Cancer
The safety and efficacy of Trastuzumab in combination with cisplatin and a fluoropyrimidine (capecitabine or 5-fluorouracil) were studied in patients previously untreated for metastatic gastric or gastroesophageal junction adenocarcinoma (Study 7). In this open-label, multi-center trial, 594 patients were randomized 1:1 to Trastuzumab in combination with cisplatin and a fluoropyrimidine (FC+H) or chemotherapy alone (FC). Randomization was stratified by extent of disease (metastatic vs. locally advanced), primary site (gastric vs. gastroesophageal junction), tumor measurability (yes vs. no), ECOG performance status (0,1 vs. 2), and fluoropyrimidine (capecitabine vs. 5-fluorouracil). All patients were either HER2 gene amplified (FISH+) or HER2 overexpressing (IHC 3+). Patients were also required to have adequate cardiac function (e.g., LVEF > 50%). On the Trastuzumab-containing arm, Trastuzumab was administered as an IV infusion at an initial dose of 8 mg/kg followed by 6 mg/kg every 3 weeks until disease progression. On both study arms cisplatin was administered at a dose of 80 mg/m2 Day 1 every 3 weeks for 6 cycles as a 2 hour IV infusion. On both study arms capecitabine was administered at 1000 mg/m2 dose orally twice daily (total daily dose 2000 mg/m2) for 14 days of each 21 day cycle for 6 cycles. Alternatively continuous intravenous infusion (CIV) 5-fluorouracil was administered at a dose of 800 mg/m2/day from Day 1 through Day 5 every three weeks for 6 cycles. The median age of the study population was 60 years (range: 21-83); 76% were male; 53% were Asian, 38% Caucasian, 5% Hispanic, 5% other racial/ethnic groups; 91% had ECOG PS of 0 or 1; 82% had primary gastric cancer and 18% had primary gastroesophageal adenocarcinoma. Of these patients, 23% had undergone prior gastrectomy, 7% had received prior neoadjuvant and/or adjuvant therapy, and 2% had received prior radiotherapy. The main outcome measure of Study 7 was overall survival (OS), analyzed by the unstratified log-rank test. The final OS analysis based on 351 deaths was statistically significant (nominal significance level of 0.0193). An updated OS analysis was conducted at one year after the final analysis. The efficacy results of both the final and the updated analyses are summarized in Table 11 and Figure 7.
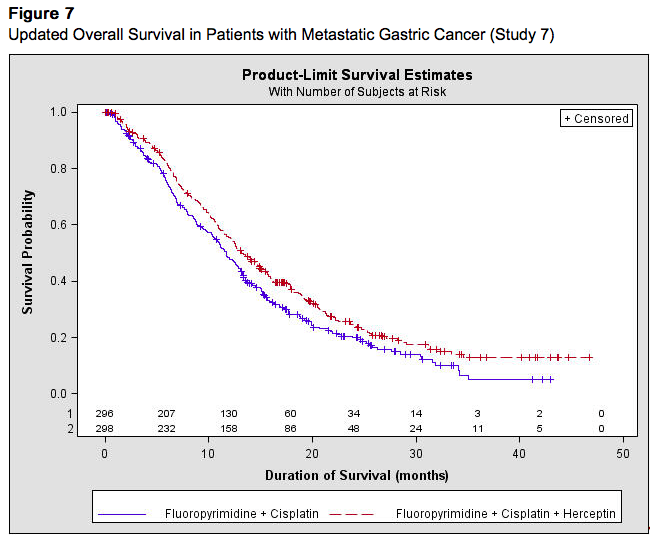
An exploratory analysis of OS in patients based on HER2 gene amplification (FISH) and protein overexpression (IHC) testing is summarized in Table 12.
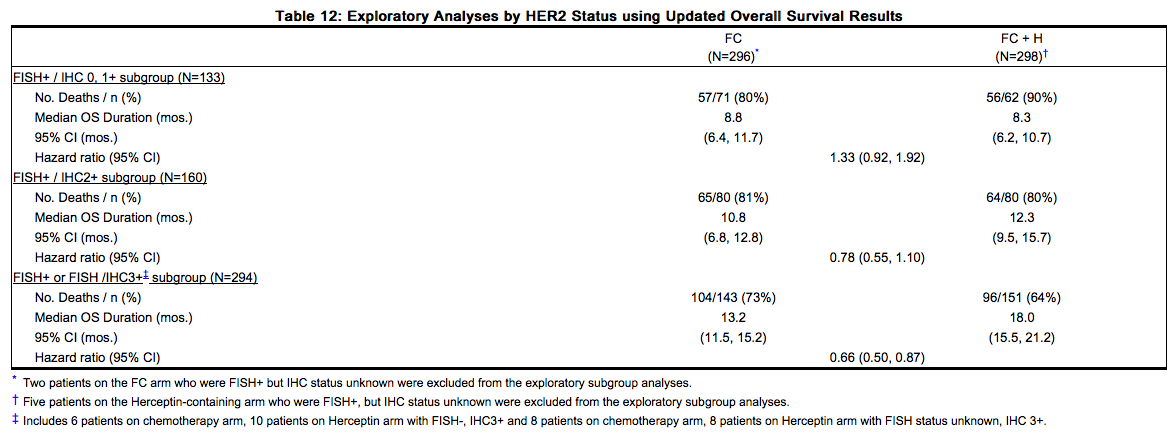
How Supplied
Trastuzumab is supplied in a multi-use vial containing 440 mg trastuzumab as a lyophilized sterile powder, under vacuum. Each carton contains one vial Trastuzumab® and one vial (20 mL) of Bacteriostatic Water for Injection (BWFI), USP, containing 1.1% benzyl alcohol as a preservative. NDC 50242-134-68.
Storage
Vials of Trastuzumab are stable at 2–8°C (36–46°F) prior to reconstitution. Do not use beyond the expiration date stamped on the vial. A vial of Trastuzumab reconstituted with BWFI, as supplied, is stable for 28 days after reconstitution when stored refrigerated at 2–8°C (36–46°F). Discard any remaining multi-dose reconstituted solution after 28 days. A vial of Trastuzumab reconstituted with unpreserved SWFI (not supplied) should be used immediately and any unused portion discarded. Do Not Freeze Trastuzumab following reconstitution or dilution. The solution of Trastuzumab for infusion diluted in polyvinylchloride or polyethylene bags containing 0.9% Sodium Chloride Injection, USP, should be stored at 2–8°C (36–46°F) for no more than 24 hours prior to use.
Images
Drug Images
{{#ask: Page Name::Trastuzumab |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
{{#ask: Label Page::Trastuzumab |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
- Advise patients to contact a health care professional immediately for any of the following: new onset or worsening shortness of breath, cough, swelling of the ankles/legs, swelling of the face, palpitations, weight gain of more than 5 pounds in 24 hours, dizziness or loss of consciousness
- Advise pregnant women and women of childbearing potential that Trastuzumab exposure can result in fetal harm
- Advise women of childbearing potential to use effective contraceptive methods during treatment and for a minimum of six months following Trastuzumab
- Advise nursing mothers treated with Trastuzumab to discontinue nursing or discontinue Trastuzumab, taking into account the importance of the drug to the mother
- Encourage women who are exposed to Trastuzumab during pregnancy to enroll in MotHER the Trastuzumab Pregnancy Registry (1-800-690-6720)
Precautions with Alcohol
Alcohol-Trastuzumab interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
Look-Alike Drug Names
Trastuzumab - Kadcyla[4]
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
- ↑ Small EJ, Bok R, Reese DM, Sudilovsky D, Frohlich M (2001). "Docetaxel, estramustine, plus trastuzumab in patients with metastatic androgen-independent prostate cancer". Semin Oncol. 28 (4 Suppl 15): 71–6. PMID 11685733.
- ↑ Zagouri F, Sergentanis TN, Bartsch R, Berghoff AS, Chrysikos D, de Azambuja E; et al. (2013). "Intrathecal administration of trastuzumab for the treatment of meningeal carcinomatosis in HER2-positive metastatic breast cancer: a systematic review and pooled analysis". Breast Cancer Res Treat. 139 (1): 13–22. doi:10.1007/s10549-013-2525-y. PMID 23588955.
- ↑ Azzoli CG, Krug LM, Miller VA, Kris MG, Mass R (2002). "Trastuzumab in the treatment of non-small cell lung cancer". Semin Oncol. 29 (1 Suppl 4): 59–65. PMID 11894015.
- ↑ "https://www.ismp.org". External link in
|title=(help)
{{#subobject:
|Label Page=Trastuzumab |Label Name=Trastuzumab_label_01.jpg
}}
{{#subobject:
|Label Page=Trastuzumab |Label Name=Trastuzumab_panel_01.png
}}
{{#subobject:
|Label Page=Trastuzumab |Label Name=Trastuzumab_panel_02.png
}}