Ivermectin (oral)
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Adeel Jamil, M.D. [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Ivermectin (oral) is a anthelmintic and anti-infective agent that is FDA approved for the treatment of intestinal (i.e., nondisseminated) strongyloidiasis due to the nematode parasite Strongyloides stercoralis and onchocerciasis due to the nematode parasite Onchocerca volvulus.. Common adverse reactions include fatigue, abdominal pain, anorexia, constipation, diarrhea, nausea, vomiting, dizziness, somnolence, vertigo, tremor, pruritus, rash and urticaria..
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
- Ivermectin is indicated for the treatment of the following infections:
- Strongyloidiasis of the intestinal tract
- Ivermectin is indicated for the treatment of intestinal (i.e., nondisseminated) strongyloidiasis due to the nematode parasite Strongyloides stercoralis.
- This indication is based on clinical studies of both comparative and open-label designs, in which 64-100% of infected patients were cured following a single 200-mcg/kg dose of Ivermectin.
- Ivermectin is indicated for the treatment of onchocerciasis due to the nematode parasite Onchocerca volvulus.
- This indication is based on randomized, double-blind, placebo-controlled and comparative studies conducted in 1427 patients in onchocerciasis-endemic areas of West Africa. The comparative studies used diethylcarbamazine citrate (DEC-C).
- NOTE: Ivermectin has no activity against adult Onchocerca volvulus parasites. The adult parasites reside in subcutaneous nodules which are infrequently palpable. Surgical excision of these nodules (nodulectomy) may be considered in the management of patients with onchocerciasis, since this procedure will eliminate the microfilariae-producing adult parasites.
Dosing Information
- The recommended dosage of Ivermectin for the treatment of strongyloidiasis is a single oral dose designed to provide approximately 200 mcg of Ivermectin per kg of body weight. See Table 1 for dosage guidelines. Patients should take tablets on an empty stomach with water. In general, additional doses are not necessary. However, follow-up stool examinations should be performed to verify eradication of infection.

Onchocerciasis
- The recommended dosage of Ivermectin for the treatment of onchocerciasis is a single oral dose designed to provide approximately 150 mcg of Ivermectin per kg of body weight. See Table 2 for dosage guidelines. Patients should take tablets on an empty stomach with water. In mass distribution campaigns in international treatment programs, the most commonly used dose interval is 12 months. For the treatment of individual patients, retreatment may be considered at intervals as short as 3 months.

Off-Label Use and Dosage (Adult)
Guideline-Supported Use
- Dosing Information
- 50 to 200 micrograms/kilogram
- Dosing Information
Non–Guideline-Supported Use
- Dosing Information
- 50 to 200 micrograms/kilogram [1]
- Dosing Information
- Dosing Information
- PO single dose of 200 µg/per kg [2]
- Dosing Information
- Dosing Information
- PO 50 to 200 micrograms/kg
- Dosing Information
- Infection by Loa loa
- Dosing Information
- PO single dose single dose of 300 micrograms/kg
- Dosing Information
- Infection by Wuchereria bancrofti
- Dosing Information
- 25 to 200 micrograms/kg, oral for 5 to 10 days
- Dosing Information
- Dosing Information
- PO single dose of 6 mg
- Dosing Information
- Dosing Information
- 200 mcg/kg PO and then repeat it within 2 weeks
- Dosing Information
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
There is limited information regarding FDA-Labeled Use of Ivermectin in pediatric patients.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Ivermectin in pediatric patients.
Non–Guideline-Supported Use
- Dosing Information
- 50 to 200 micrograms/kilogram [1]
- Dosing Information
Contraindications
- Ivermectin is contraindicated in patients who are hypersensitive to any component of this product.
Warnings
- Historical data have shown that microfilaricidal drugs, such as diethylcarbamazine citrate (DEC-C), might cause cutaneous and/or systemic reactions of varying severity (the Mazzotti reaction) and ophthalmological reactions in patients with onchocerciasis. These reactions are probably due to allergic and inflammatory responses to the death of microfilariae. Patients treated with Ivermectin for onchocerciasis may experience these reactions in addition to clinical adverse reactions possibly, probably, or definitely related to the drug itself.
- The treatment of severe Mazzotti reactions has not been subjected to controlled clinical trials. Oral hydration, recumbency, intravenous normal saline, and/or parenteral corticosteroids have been used to treat postural hypotension. Antihistamines and/or aspirin have been used for most mild to moderate cases.
PRECAUTIONS
- General
- After treatment with microfilaricidal drugs, patients with hyperreactive onchodermatitis (sowda) may be more likely than others to experience severe adverse reactions, especially edema and aggravation of onchodermatitis.
- Rarely, patients with onchocerciasis who are also heavily infected with Loa loa may develop a serious or even fatal encephalopathy either spontaneously or following treatment with an effective microfilaricide. In these patients, the following adverse experiences have also been reported: pain (including neck and back pain), red eye, conjunctival hemorrhage, dyspnea, urinary and/or fecal incontinence, difficulty in standing/walking, mental status changes, confusion, lethargy, stupor, seizures, or coma. This syndrome has been seen very rarely following the use of Ivermectin. In individuals who warrant treatment with Ivermectin for any reason and have had significant exposure to Loa loa-endemic areas of West or Central Africa, pretreatment assessment for loiasis and careful post-treatment follow-up should be implemented.
Adverse Reactions
Clinical Trials Experience
Strongyloidiasis
- In four clinical studies involving a total of 109 patients given either one or two doses of 170 to 200 mcg/kg of Ivermectin, the following adverse reactions were reported as possibly, probably, or definitely related to Ivermectin:
Body as a Whole:
- Asthenia/fatigue (0.9%), abdominal pain (0.9%)
Gastrointestinal:
- Anorexia (0.9%), constipation (0.9%), diarrhea (1.8%), nausea (1.8%), vomiting (0.9%)
Nervous System/Psychiatric:
- Dizziness (2.8%), somnolence (0.9%), vertigo (0.9%), tremor (0.9%)
Skin:
- In comparative trials, patients treated with Ivermectin experienced more abdominal distention and chest discomfort than patients treated with albendazole. However, Ivermectin was better tolerated than thiabendazole in comparative studies involving 37 patients treated with thiabendazole.
- The Mazzotti-type and ophthalmologic reactions associated with the treatment of onchocerciasis or the disease itself would not be expected to occur in strongyloidiasis patients treated with Ivermectin.
Laboratory Test Findings
- In clinical trials involving 109 patients given either one or two doses of 170 to 200 mcg/kg Ivermectin, the following laboratory abnormalities were seen regardless of drug relationship: elevation in ALT and/or AST (2%), decrease in leukocyte count (3%). Leukopenia and anemia were seen in one patient.
Onchocerciasis
- In clinical trials involving 963 adult patients treated with 100 to 200 mcg/kg Ivermectin, worsening of the following Mazzotti reactions during the first 4 days post-treatment were reported: arthralgia/synovitis (9.3%), axillary lymph node enlargementand tenderness (11.0% and 4.4%, respectively), cervical lymph node enlargement and tenderness (5.3% and 1.2%, respectively), inguinal lymph node enlargement and tenderness (12.6% and 13.9%, respectively), other lymph node enlargement and tenderness (3.0% and 1.9%, respectively), pruritus (27.5%), skin involvement including edema, papular and pustular or frank urticarial rash (22.7%), and fever (22.6%).
- In clinical trials, ophthalmological conditions were examined in 963 adult patients before treatment, at day 3, and months 3 and 6 after treatment with 100 to 200 mcg/kg Ivermectin. Changes observed were primarily deterioration from baseline 3 days post-treatment. Most changes either returned to baseline condition or improved over baseline severity at the month 3 and 6 visits. The percentages of patients with worsening of the following conditions at day 3, month 3 and 6, respectively, were: limbitis: 5.5%, 4.8%, and 3.5% and punctate opacity: 1.8%, 1.8%, and 1.4%. The corresponding percentages for patients treated with placebo were: limbitis: 6.2%, 9.9%, and 9.4% and punctate opacity: 2.0%, 6.4%, and 7.2%.
- In clinical trials involving 963 adult patients who received 100 to 200 mcg/kg Ivermectin, the following clinical adverse reactions were reported as possibly, probably, or definitely related to the drug in ≥1% of the patients: facial edema (1.2%), peripheral edema (3.2%), orthostatic hypotension (1.1%), and tachycardia (3.5%). Drug-related headache and myalgia occurred in <1% of patients (0.2% and 0.4%, respectively). However, these were the most common adverse experiences reported overall during these trials regardless of causality (22.3% and 19.7%, respectively).
- A similar safety profile was observed in an open study in pediatric patients ages 6 to 13.
- The following ophthalmological side effects do occur due to the disease itself but have also been reported after treatment with Ivermectin: abnormal sensation in the eyes, eyelid edema, anterior uveitis, conjunctivitis, limbitis, keratitis, and chorioretinitis or choroiditis. These have rarely been severe or associated with loss of vision and have generally resolved without corticosteroid treatment.
Laboratory Test Findings
- In controlled clinical trials, the following laboratory adverse experiences were reported as possibly, probably, or definitely related to the drug in ≥1% of the patients: eosinophilia (3%) and hemoglobin increase (1%).
Postmarketing Experience
- The following adverse reactions have been reported since the drug was registered overseas:
Onchocerciasis
All Indications
- Hypotension (mainly orthostatic hypotension), worsening of bronchial asthma, toxic epidermal necrolysis, Stevens-Johnson syndrome, seizures, hepatitis, elevation of liver enzymes, and elevation of bilirubin.
Drug Interactions
- Post-marketing reports of increased INR (International Normalized Ratio) have been rarely reported when Ivermectin was co-administered with warfarin.
Use in Specific Populations
Pregnancy
- Ivermectin has been shown to be teratogenic in mice, rats, and rabbits when given in repeated doses of 0.2, 8.1, and 4.5 times the maximum recommended human dose, respectively (on a mg/m2/day basis). Teratogenicity was characterized in the three species tested by cleft palate; clubbed forepaws were additionally observed in rabbits. These developmental effects were found only at or near doses that were maternotoxic to the pregnant female. Therefore, Ivermectin does not appear to be selectively fetotoxic to the developing fetus. There are, however, no adequate and well-controlled studies in pregnant women. Ivermectin should not be used during pregnancy since safety in pregnancy has not been established.
- Australian Drug Evaluation Committee (ADEC) Pregnancy Category
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Ivermectin in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Ivermectin during labor and delivery.
Nursing Mothers
- Ivermectin is excreted in human milk in low concentrations. Treatment of mothers who intend to breast-feed should only be undertaken when the risk of delayed treatment to the mother outweighs the possible risk to the newborn.
Pediatric Use
- Safety and effectiveness in pediatric patients weighing less than 15 kg have not been established.
Geriatic Use
- Clinical studies of Ivermectin did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects. Other reported clinical experience has not identified differences in responses between the elderly and younger patients. In general, treatment of an elderly patient should be cautious, reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy.
Gender
There is no FDA guidance on the use of Ivermectin with respect to specific gender populations.
Race
There is no FDA guidance on the use of Ivermectin with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Ivermectin in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Ivermectin in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Ivermectin in women of reproductive potentials and males.
Immunocompromised Patients
- In immunocompromised (including HIV-infected) patients being treated for intestinal strongyloidiasis, repeated courses of therapy may be required. Adequate and well-controlled clinical studies have not been conducted in such patients to determine the optimal dosing regimen. Several treatments, i.e., at 2-week intervals, may be required, and cure may not be achievable. Control of extra-intestinal strongyloidiasis in these patients is difficult, and suppressive therapy, i.e., once per month, may be helpful.
Administration and Monitoring
Administration
Monitoring
There is limited information regarding Monitoring of Ivermectin in the drug label.
- Description
IV Compatibility
There is limited information regarding IV Compatibility of Ivermectin in the drug label.
Overdosage
- Significant [[lethal|lethality]] was observed in mice and rats after single oral doses of 25 to 50 mg/kg and 40 to 50 mg/kg, respectively. No significant lethality was observed in dogs after single oral doses of up to 10 mg/kg. At these doses, the treatment-related signs that were observed in these animals include ataxia, bradypnea, tremors, ptosis, decreased activity, emesis, and mydriasis.
- In accidental intoxication with, or significant exposure to, unknown quantities of veterinary formulations of Ivermectin in humans, either by ingestion, inhalation, injection, or exposure to body surfaces, the following adverse effects have been reported most frequently: rash, edema, headache, dizziness, asthenia, nausea, vomiting, and diarrhea. Other adverse effects that have been reported include: seizure, ataxia, dyspnea, abdominal pain, paresthesia, urticaria, and contact dermatitis.
- In case of accidental poisoning, supportive therapy, if indicated, should include parenteral fluids and electrolytes, respiratory support (oxygen and mechanical ventilation if necessary) and pressor agents if clinically significant hypotension is present. Induction of emesis and/or gastric lavage as soon as possible, followed by purgatives and other routine anti-poison measures, may be indicated if needed to prevent absorption of ingested material.
Pharmacology
| |
Ivermectin (oral)
| |
| Systematic (IUPAC) name | |
| 22,23-dihydroavermectin B1a + 22,23-dihydroavermectin B1b | |
| Identifiers | |
| CAS number | 71827-03-7 |
| ATC code | P02 Template:ATCvet Template:ATCvet |
| PubChem | |
| DrugBank | |
| Chemical data | |
| Formula | Template:OrganicBox atomTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox |
| Mol. mass | 875.10 g/mol |
| Pharmacokinetic data | |
| Bioavailability | ? |
| Protein binding | 93% |
| Metabolism | Liver (CYP450) |
| Half life | 18 hours |
| Excretion | Feces; <1% urine |
| Therapeutic considerations | |
| Pregnancy cat. | |
| Legal status |
[[Prescription drug|Template:Unicode-only]](US) |
| Routes | Oral, topical |
Mechanism of Action
Microbiology
- Ivermectin is a member of the avermectin class of broad-spectrum antiparasitic agents which have a unique mode of action. Compounds of the class bind selectively and with high affinity to glutamate-gated chloride ion channels which occur in invertebrate nerve and muscle cells. This leads to an increase in the permeability of the cell membrane to chloride ions with hyperpolarization of the nerve or muscle cell, resulting in paralysis and death of the parasite. Compounds of this class may also interact with other ligand-gated chloride channels, such as those gated by the neurotransmitter gamma-aminobutyric acid (GABA).
- The selective activity of compounds of this class is attributable to the facts that some mammals do not have glutamate-gated chloride channels and that the avermectins have a low affinity for mammalian ligand-gated chloride channels. In addition, Ivermectin does not readily cross the blood-brain barrier in humans.
- Ivermectin is active against various life-cycle stages of many but not all nematodes. It is active against the tissue microfilariae of Onchocerca volvulus but not against the adult form. Its activity against Strongyloides stercoralis is limited to the intestinal stages.
Structure
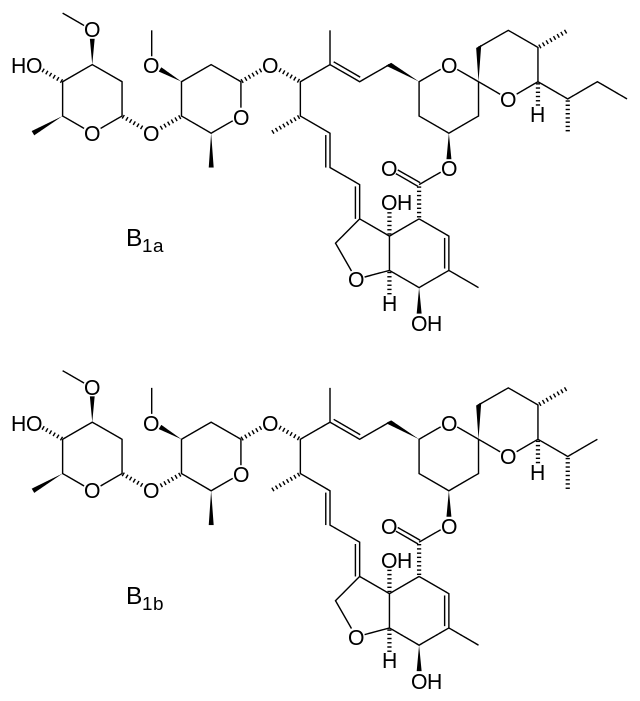
- Ivermectin is a semisynthetic, anthelmintic agent for oral administration. Ivermectin is derived from the avermectins, a class of highly active broad-spectrum, anti-parasitic agents isolated from the fermentation products of Streptomyces avermitilis. Ivermectin is a mixture containing at least 90% 5-O-demethyl-22,23-dihydroavermectin A1a and less than 10% 5-O-demethyl-25-de(1-methylpropyl)-22,23-dihydro-25-(1-methylethyl)avermectin A1a, generally referred to as 22,23-dihydroavermectin B1a and B1b, or H2B1a and H2B1b, respectively. The respective empirical formulas are C48H74O14 and C47H72O14, with molecular weights of 875.10 and 861.07, respectively. The structural formulas are:

Pharmacodynamics
There is limited information regarding Pharmacodynamics of Ivermectin in the drug label.
Pharmacokinetics
- Following oral administration of Ivermectin, plasma concentrations are approximately proportional to the dose. In two studies, after single 12-mg doses of Ivermectin in fasting healthy volunteers (representing a mean dose of 165 mcg/kg), the mean peak plasma concentrations of the major component (H2B1a) were 46.6 (±21.9) (range: 16.4-101.1) and 30.6 (±15.6) (range: 13.9-68.4) ng/mL, respectively, at approximately 4 hours after dosing. Ivermectin is metabolized in the liver, and Ivermectin and/or its metabolites are excreted almost exclusively in the feces over an estimated 12 days, with less than 1% of the administered dose excreted in the urine. The plasma half-life of Ivermectin in man is approximately 18 hours following oral administration.
- The safety and pharmacokinetic properties of Ivermectin were further assessed in a multiple-dose clinical pharmacokinetic study involving healthy volunteers. Subjects received oral doses of 30 to 120 mg (333 to 2000 mcg/kg) Ivermectin in a fasted state or 30 mg (333 to 600 mcg/kg) Ivermectin following a standard high-fat (48.6 g of fat) meal. Administration of 30 mg Ivermectin following a high-fat meal resulted in an approximate 2.5-fold increase in bioavailability relative to administration of 30 mg Ivermectin in the fasted state.
- In vitro studies using human liver microsomes and recombinant CYP450 enzymes have shown that Ivermectin is primarily metabolized by CYP3A4. Depending on the in vitro method used, CYP2D6 and CYP2E1 were also shown to be involved in the metabolism of Ivermectin but to a significantly lower extent compared to CYP3A4. The findings of in vitro studies using human liver microsomes suggest that clinically relevant concentrations of Ivermectin do not significantly inhibit the metabolizing activities of CYP3A4, CYP2D6, CYP2C9, CYP1A2, and CYP2E1.
Nonclinical Toxicology
There is limited information regarding Nonclinical Toxicology of Ivermectin in the drug label.
Clinical Studies
Strongyloidiasis]]=
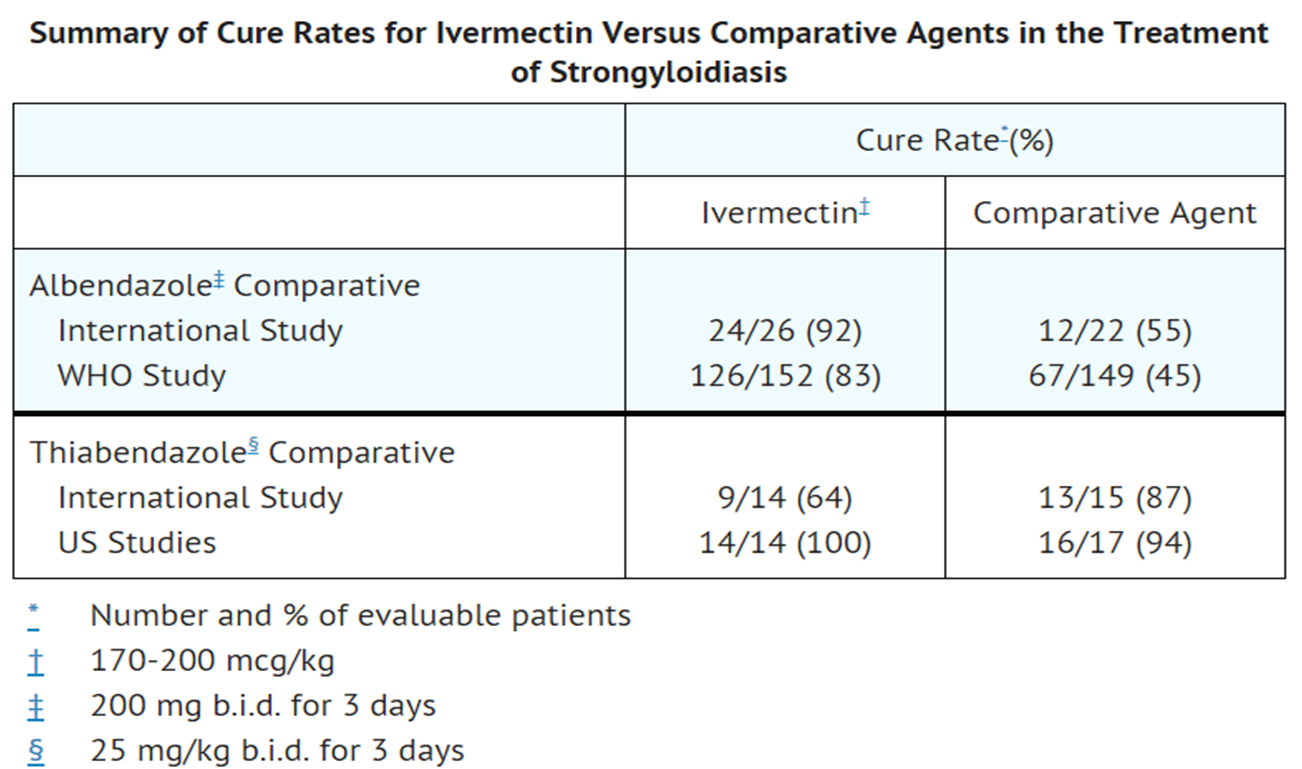
- Two controlled clinical studies using albendazole as the comparative agent were carried out in international sites where albendazole is approved for the treatment of strongyloidiasis of the gastrointestinal tract, and three controlled studies were carried out in the U.S. and internationally using thiabendazole as the comparative agent. Efficacy, as measured by cure rate, was defined as the absence of larvae in at least two follow-up stool examinations 3 to 4 weeks post-therapy. Based on this criterion, efficacy was significantly greater for Ivermectin (a single dose of 170 to 200 mcg/kg) than for albendazole (200 mg b.i.d. for 3 days). Ivermectin administered as a single dose of 200 mcg/kg for 1 day was as efficacious as thiabendazole administered at 25 mg/kg b.i.d. for 3 days.
- In one study conducted in France, a non-endemic area where there was no possibility of reinfection, several patients were observed to have recrudescence of Strongyloides larvae in their stool as long as 106 days following Ivermectin therapy. Therefore, at least three stool examinations should be conducted over the three months following treatment to ensure eradication. If recrudescence of larvae is observed, retreatment with Ivermectin is indicated. Concentration techniques (such as using a Baermann apparatus) should be employed when performing these stool examinations, as the number of Strongyloides larvae per gram of feces may be very low.
Onchocerciasis
- The evaluation of Ivermectin in the treatment of onchocerciasis is based on the results of clinical studies involving 1278 patients. In a double-blind, placebo-controlled study involving adult patients with moderate to severe onchocercal infection, patients who received a single dose of 150 mcg/kg Ivermectin experienced an 83.2% and 99.5% decrease in skin microfilariae count (geometric mean) 3 days and 3 months after the dose, respectively. A marked reduction of >90% was maintained for up to 12 months after the single dose. As with other microfilaricidal drugs, there was an increase in the microfilariae count in the anterior chamber of the eye at day 3 after treatment in some patients. However, at 3 and 6 months after the dose, a significantly greater percentage of patients treated with Ivermectin had decreases in microfilariae count in the anterior chamber than patients treated with placebo.
- In a separate open study involving pediatric patients ages 6 to 13 (n=103; weight range: 17-41 kg), similar decreases in skin microfilariae counts were observed for up to 12 months after dosing.
Carcinogenesis, Mutagenesis, Impairment of Fertility
- Long-term studies in animals have not been performed to evaluate the carcinogenic potential of Ivermectin.
- Ivermectin was not genotoxic in vitro in the Ames microbial mutagenicity assay of Salmonella typhimurium strains TA1535, TA1537, TA98, and TA100 with and without rat liver enzyme activation, the Mouse Lymphoma Cell Line L5178Y (cytotoxicity and mutagenicity) assays, or the unscheduled DNA synthesis assay in human fibroblasts.
- Ivermectin had no adverse effects on the fertility in rats in studies at repeated doses of up to 3 times the maximum recommended human dose of 200 mcg/kg (on a mg/m2/day basis).
How Supplied
No. 8495 — Tablets STROMECTOL 3 mg are white, round, flat, bevel-edged tablets coded MSD on one side and 32 on the other side. They are supplied as follows:
NDC 0006-0032-20 unit dose packages of 20.
Dist. by: Merck Sharp & Dohme Corp., a subsidiary of MERCK & CO., INC., Whitehouse Station, NJ 08889, USA
Manufactured by: Merck Sharp & Dohme BV Waarderweg 39 2031 BN Haarlem Netherlands
Issued May 2010
Printed in the Netherlands
9032319
87447/080610
8495
Storage
- Store at temperatures below 30°C (86°F).
Images
Drug Images
{{#ask: Page Name::Ivermectin (oral) |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
Stromectol® 3 mg (Ivermectin Tablets)
Manuf. by: Merck Sharp & Dohme BV Waarderweg 39 2031 BN Haarlem, Netherlands
Formulated in Netherlands
Dist. by: Merck Sharp & Dohme Corp., a subsidiary of MERCK & CO., INC. Whitehouse Station, NJ 08889, USA
Each tablet contains 3 mg of Ivermectin.
USUAL DOSAGE: See accompanying circular.
Store at temperatures below 30°C (86°F).
Rx only
20 Tablets (2 Foil Strips of 10 tablets each)
NDC 0006-0032-20
This is a bulk package and not intended for dispensing. The aluminum foil strip is not child resistant. Remove tablets from aluminum foil strip and dispense tablets in appropriate container.
20 {{#ask: Label Page::Ivermectin (oral) |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
- Ivermectin should be taken on an empty stomach with water.
- Strongyloidiasis: The patient should be reminded of the need for repeated stool examinations to document clearance of infection with Strongyloides stercoralis.
- onchocerciasis: The patient should be reminded that treatment with Ivermectin does not kill the adult Onchocerca parasites, and therefore repeated follow-up and retreatment is usually required.
Precautions with Alcohol
- Alcohol-Ivermectin interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
- Stromectol®
- Sklice®
Look-Alike Drug Names
There is limited information regarding Ivermectin (oral) Look-Alike Drug Names in the drug label.
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
- ↑ 1.0 1.1 Marti H, Haji HJ, Savioli L, Chwaya HM, Mgeni AF, Ameir JS; et al. (1996). "A comparative trial of a single-dose ivermectin versus three days of albendazole for treatment of Strongyloides stercoralis and other soil-transmitted helminth infections in children". Am J Trop Med Hyg. 55 (5): 477–81. PMID 8940976.
- ↑ Bouchaud O, Houzé S, Schiemann R, Durand R, Ralaimazava P, Ruggeri C; et al. (2000). "Cutaneous larva migrans in travelers: a prospective study, with assessment of therapy with ivermectin". Clin Infect Dis. 31 (2): 493–8. doi:10.1086/313942. PMID 10987711.
{{#subobject:
|Page Name=Ivermectin (oral)
|Pill Name=No image.jpg
|Drug Name=
|Pill Ingred=|+sep=;
|Pill Imprint=
|Pill Dosage={{{dosageValue}}} {{{dosageUnit}}}
|Pill Color=|+sep=;
|Pill Shape=
|Pill Size (mm)=
|Pill Scoring=
|Pill Image=
|Drug Author=
|NDC=
}}
{{#subobject:
|Label Page=Ivermectin (oral) |Label Name=Ivermectin package label01.png
}}
{{#subobject:
|Label Page=Ivermectin (oral) |Label Name=Ivermectin package label02.png
}}