Failed back syndrome
| Failed back syndrome |
Please Take Over This Page and Apply to be Editor-In-Chief for this topic: There can be one or more than one Editor-In-Chief. You may also apply to be an Associate Editor-In-Chief of one of the subtopics below. Please mail us [1] to indicate your interest in serving either as an Editor-In-Chief of the entire topic or as an Associate Editor-In-Chief for a subtopic. Please be sure to attach your CV and or biographical sketch.
Overview
Failed back syndrome or post-laminectomy syndrome is a condition characterized by persistent pain following back surgeries.
Failed back syndrome (FBS), also called "failed back surgery syndrome" (FBSS), refers to chronic back and/or leg pain that occurs after back (spinal) surgery.[1][2] It is characterized as a chronic pain syndrome. Multiple factors can contribute to the onset or development of FBS. Contributing factors include but are not limited to residual or recurrent disc herniation, persistent post-operative pressure on a spinal nerve, altered joint mobility, joint hypermobility with instability, scar tissue (fibrosis), depression, anxiety, sleeplessness and spinal muscular deconditioning. An individual may be predisposed to the development of FBS due to systemic disorders such as diabetes, autoimmune disease and peripheral blood vessels (vascular) disease. Smoking is a risk for poor recovery.
Common symptoms associated with FBS include diffuse, dull and aching pain involving the back and/or legs. Abnormal sensibility may include sharp, pricking, and stabbing pain in the extremities. The term “post-laminectomy syndrome” is used by some doctors to indicate the same condition as failed back syndrome.
The treatments of post-laminectomy syndrome include physical therapy, minor nerve blocks, transcutaneous electrical nerve stimulation (TENS), behavioral medicine, non-steroidal anti-inflammatory (NSAID) medications, membrane stabilizers, antidepressants, spinal cord stimulation, and intracathecal morphine pump. Use of epidural steroid injections may be minimally helpful in some cases. The targeted anatomic use of a potent anti-inflammatory anti-TNF therapeutics is being investigated.
The amount of spinal surgery varies around the world. The most is performed in the United States and Holland. The least in the United Kingdom and Sweden. Recently, there have been calls for more aggressive surgical treatment in Europe (see infra). Success rates of spinal surgery vary for many reasons. [3] [4]
Etiology
Patients who have undergone one or more operations on the lumbar spine, and continue to experience and report pain afterward can be divided into two groups. The first group are those in whom surgery was never indicated, or the surgery performed was never likely to achieve the desired result; and those in whom the surgery was indicated, but which technically did not achieve the intended result. [5] It has been observed that patients who have a predominant painful presentation in a radicular pattern will have a better result than those who have predominant complaints of back pain. Litigation tends to decrease the successful results of all spinal surgery. This includes personal injury cases (tort) and worker’s compensation cases. [6] [7]
The second group includes patients who had incomplete or inadequate operations. Lumbar spinal stenosis may be overlooked, especially when it is associated with disc protrusion or herniation. Removal of a disc, while not addressing the underlying presence of stenosis can lead to disappointing results. [8] Occasionally operating on the wrong level occurs, as does failure to recognize an extruded or sequestered disc fragment. Inadequate or inappropriate surgical exposure can lead to other problems in not getting to the underlying pathology. Hakelius reported a 3% incidence of serious nerve root damage. [9]
In 1992, Turner et al. [10] published a survey of 74 articles on the results after decompression for spinal stenosis. Good to excellent results were on average reported by 64% of the patients. There was, however, a wide variation in outcomes reported. There was a better result in patients who had a degenerative spondylolishesis. A similarly desigined study by Mardjekto et al. [11] found that a concomitant spinal arthrodesis (fusion) had a greater success rate. Herron and Trippi [12] evaluated 24 patients, all with degenerative spondylolisthesis treated with laminectomy alone. At follow-up varying between 18 to 71 months after surgery, 20 out of 24 (83%) patients reported a good result. Epstein [13] reported on 290 patients treated over a 25 year period. Excellent results were obtained in 69% and good results in 13%. However, these optimistic reports do not correlate with "return to competitive employment" rates, which for the most part are dismal in post spinal surgery series. To be honest, most articles surverying surgical success do not report on return to work.
In the past two decades there has been a dramatic increase in fusion surgery in the U.S.: in 2001 over 122,000 lumbar fusions were performed, a 22% increase from 1990 in fusions per 100,000 population, increasing to an estimate of 250,000 in 2003, and 500,000 in 2006.[14][15][16] In 2003, the national bill for the hardware for fusion alone was estimated to have soared to $2.5 billion a year.[15] [17] For patients with continued pain after surgery which is not due to the above complications or conditions, interventional pain physicians speak of the need to identify the "pain generator" i.e. the anatomical structure responsible for the patient's pain. To be effective, the surgeon must operate on the correct anatomic structure; however it is often not possible to determine the source of the pain.[18][19] The reason for this is that many patients with chronic pain often have disc bulges at multiple spinal levels and the physical examination and imaging studies are unable to pinpoint the source of pain.[18] In addition, spinal fusion itself, particularly if more than one spinal level is operated on, may result in “adjacent segment degeneration”.[20] This is thought to occur because the fused segments may result in increased torsional and stress forces being transmitted to the intervertebral discs located above and below the fused vertebrae.[20] This pathology is one reason behind the development of artificial discs as a possible alternative to fusion surgery. But the fusion surgeons argue, with some validity, that spinal fusion is more time-tested, and artificial discs contain metal hardware that is unlikely to last as long as biological material without shattering and leaving metal fragments in the spinal canal. These represent different schools of thought.
Another highly relevant consideration is the increasing recognition of the importance of “chemical radiculitis” in the generation of back pain.[21] A primary focus of surgery is to remove “pressure” or reduce mechanical compression on a neural element: either the spinal cord, or a nerve root. But it is increasingly recognized that back pain, rather than being solely due to compression, may instead entirely be due to chemical inflammation of the nerve root. It has been known for several decades that disc herniations result in a massive inflammation of the associated nerve root.[22][23] [24][21] In the past five years increasing evidence has pointed to a specific inflammatory mediator of this pain.[25][26] This inflammatory molecule, called tumor necrosis factor-alpha (TNF), is released not only by the herniated or protruding disc, but also in cases of disc tear (annular tear), by facet joints, and in spinal stenosis.[21][27][28][29] In addition to causing pain and inflammation, TNF may also contribute to disc degeneration.[30] If the cause of the pain is not compression, but rather is inflammation mediated by TNF, then this may well explain why surgery might not relieve the pain, and might even exacerbate it, resulting in FBSS.
Patient selection
Patients who have sciatic pain (pain in the back, radiating down the buttock to the leg) and clear clinical findings of an identifiable radicular nerve loss caused by a herniated disc will have a better post operative course that those who simply have low back pain. If a specific disc herniation causing pressure on a nerve root cannot be identified, the results of surgery are likely to be disappointing. Patients involved in worker’s compensation, tort litigation or other compensation systems tend to fare more poorly after surgery. Surgery for spinal stenosis usually has a good outcome, if the surgery is done in an extensive manner, and done within the first year or so of the appearance of symptoms. [31] [32] [33] [34] [35]
Oaklander and North define the Failed Back Syndrome as a chronic pain patient after one or more surgical procedure to the spine. They delineated these characteristics of the relation between the patient and the surgeon:
(1) The patient makes increasing demands on the surgeon for pain relief. The surgeon may feels a strong responsibility to provide a remedy when the surgery has not achieved the desired goals.
(2) The patient grows increasingly angry at the failure and may become litigious.
(3) There is an escalation of narcotic pain medication which is habituating or addictive.
(4) In the face of expensive conservative treatments which are likely to fail, the surgeon is persuaded to attempt further surgery, even though this is likely to fail as well.
(5) The probability of returning to gainful employment decreases with increasing length of disability.
(6) The financial incentives to remain disabled far outweigh the incentive to recover. [36]
In the absence of a generous or comfortable economic package for disability or worker’s compensation, other psychological features may limit the ability of the patient to recover from surgery. Some patients are simply unfortunate, and fall into the category of “chronic pain” despite their desire to recover and the best efforts of the physicians involved in their care. [37] [38] [39] [40] [41] [42] [43] [44] [45] [46] [47] Even less invasive forms of surgery are not uniformly successful; approximately 30,000-40,000 laminectomy patients obtain either no relief of symptomatology or a recurrence of symptoms.[48]
Another less invasive form of spinal surgery, percutaneous disc surgery, has reported revision rates as high as 65%.[49] It is no surprise, therefore, that FBSS is a significant medical concern which merits further research and attention by the medical and surgical communities.[18][19]
Pathology
Before the advent of CT scanning, the pathology in failed back syndrome was difficult to understand. Computerized tomography in conjunction with metrizamide myelography in the late 1960s and 1970s allowed direct observation of the mechanisms involved in post operative failures. Six distinct pathologic conditions were identified:
- Recurrent or persistent disc herniation
- Spinal stenosis
- Epidural post-operative fibrosis
- Adhesive arachnoiditis
- Nerve Injury
- Pathologic location
Recurrent or persistent disc herniation
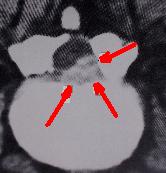
Removal of a disc at one level can lead to disc herniation at the same level or a different level at a later time. Even the most complete surgical excision of the disc still leaves 30-40% of the disc, which cannot be safely removed. This retained disc can re-herniate sometime after surgery. Virtually every major structure in the abdomen and posterior retroperitoneal canal has been injured removing discs from posterior laminetomy/discectomy surgical procedures. The most prominent of these is a laceration of the left internal iliac vein which lies in close proximity to the anterior portion of the disc. [50] [51] In some studies, recurrent pain in the same radicular pattern or a different pattern can be as high as 50% after disc surgery. [52] [53]
Many observers have noted that the most common cause of a failed back syndrome is caused from recurrent disc herniation at the same level originally operated. A rapid removal in a second surgery can be curative. The clinical picture of a recurrent disc herniation usually involves a significant pain free interval. However, physical findings may be lacking, and a good history is necessary. [54] [55] [56] [57] The time period for the emergence of new symptoms can be short or long. Diagnostic signs such as the straight leg raising test may be negative even if real pathology is present. [58] [59] The presence of a positive myelogram may represent a new disc herniation, but can also be indicative of a post operative scarring situation simply mimicking a new disc. Newer MRI imaging techniques have clarified this dilemma somewhat. [60] [61] [62] [63] [64] [65]
Conversely, a recurrent disc can be difficult to detect in the presence of post op scarring. Myelography is inadequate to completely evaluate the patient for recurrent disc disease, and CT or MRI scanning is necessary. Measurement of tissue density can be helpful. [66] [67] [68] [69] [70]
Spinal stenosis
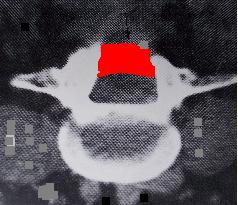
Spinal stenosis can be a late complication after laminectomy for disc herniation or when surgery was performed for the primary pathologic condition of spinal stenosis. [71] [72] [73]
In the Maine Study, among patients with lumbar spinal stenosis completing 8- to 10-year follow-up, low back pain relief, predominant symptom improvement, and satisfaction with the current state were similar in patients initially treated surgically or nonsurgically. However, leg pain relief and greater back-related functional status continued to favor those initially receiving surgical treatment. [74]
A large study of spinal stenosis from Finland found the prognostic factors for ability to work after surgery were ability to work before surgery, age under 50 years, and no prior back surgery. The very long-term outcome (mean followup time of 12.4 years) was excellent-to-good in 68% of patients (59% women and 73% men). Furthermore, in the longitudinal follow-up, the result improved between 1985 and 1991. No special complications were manifested during this very long-term follow-up time. The patients with total or subtotal block in preoperative myelography achieved the best result. Furthermore, patients with block stenosis improved their result significantly in the longitudinal follow-up.
The postoperative stenosis seen in computed tomography (CT) scans was observed in 65% of 90 patients, and it was severe in 23 patients (25%). However, this successful or unsuccessful surgical decompression did not correlate with patients' subjective disability, walking capacity or severity of pain. Previous back surgery had a strong worsening effect on surgical results. This effect was very clear in patients with total block in the preoperative myelography. The surgical result of a patient with previous back surgery was similar to that of a patient without previous back surgery when the time interval between the last two operations was more than 18 months. [75]
Post-operative MRI findings of stenosis are probably of limited value compared to symptoms experienced by patients. Patients' perception of improvement had a much stronger correlation with long-term surgical outcome than structural findings seen on postoperation magnetic resonance imaging. Degenerative findings had a greater effect on patients' walking capacity than stenotic findings [76] [77]
Postoperative radiologic stenosis was very common in patients operated on for lumbar spinal stenosis, but this did not correlate with clinical outcome. The clinician must be cautious when reconciling clinical symptoms and signs with postoperative computed tomography findings in patients operated on for lumbar spinal stenosis. [78]
A study from Georgetown University reported on one-hundred patients who had undergone decompressive surgery for lumbar stenosis between 1980 and 1985. Four patients with postfusion stenosis were included. A 5-year follow-up period was achieved in 88 patients. The mean age was 67 years, and 80% were over 60 years of age. There was a high incidence of coexisting medical diseases, but the principal disability was lumbar stenosis with neurological involvement. Initially there was a high incidence of success, but recurrence of neurological involvement and persistence of low-back pain led to an increasing number of failures. By 5 years this number had reached 27% of the available population pool, suggesting that the failure rate could reach 50% within the projected life expectancies of most patients. Of the 26 failures, 16 were secondary to renewed neurological involvement, which occurred at new levels of stenosis in eight and recurrence of stenosis at operative levels in eight. Reoperation was successful in 12 of these 16 patients, but two required a third operation. The incidence of spondylolisthesis at 5 years was higher in the surgical failures (12 of 26 patients) than in the surgical successes (16 of 64). Spondylolisthetic stenosis tended to recur within a few years following decompression. Because of age and associated illnesses, fusion may be difficult to achieve in this group. [79]
Post operative infection
A small minority of lumbar surgical patients will develop a post operative infection. In most cases, this is a bad complication and does not bode well for eventual improvement or future employability. Reports from the surgical literature indicate an infection rate anywhere from 0% to almost 12%. [80] [81] [82] [83] [84] [85] [86] [87] [88] [89] [90] [91] [92] [93] [94] [95] The incidence of infection tends to increase as the complexity of the procedure and operating time increase. Usage of metal implants (instrumentation) tends to increase the risk of infection. Factors associated with an increased infection include diabetes mellitus, obesity, malnutrition, smoking, previous infection, rheumatoid arthritis, and immunodeficiency. [96] [97] [98] [99] [100] [101]
Epidural post-operative fibrosis
Epidural scarring following a laminectomy for disc excision is a common feature when re-operating for recurrent sciatica or radiculopathy. [102] When the scarring is associated with a disc herniation and/or recurrent spinal stenosis, it is relatively common, occurring in more than 60% of cases. For a time, it was theorized that placing a fat graft over the dural could prevent post operative scarring. However, initial enthusiasm has waned in recent years. [103] [104] [105] [106] [107] In an extensive laminectomy involving 2 or more vertebra, post operative scarring is the norm. It is most often seen around the L5 and S1 nerve roots. [108] [109] [110]
Adhesive arachnoiditis
Fibrous scarring can also be a complication within the subarachnoid space. It is notoriously difficult to detect and evaluate. Prior to the development of magnetic resonance imaging, the only way to ascertain the presence of arachnoiditis was by opening the dura. In the days of CT scanning and Pantopaque and later, Metrizamide myelography, the presence of arachnoiditis could be speculated based on radiographic findings. Often, myelogpaphy prior to the introduction of Metrizamide was the cause of arachnoiditis. It can also be caused by the long term pressure brought about with either a disc herniation or spinal stenosis. [111] [112] [113] [114] [115] The presence of both epidural scarring and arachnoiditis in the same patient are probably quite common.
Arachnoiditis is a broad term denoting inflammation of the meninges and subarachnoid space. A variety of etiologies exist, including infectious, inflammatory, and neoplastic processes.
Infectious etiologies include bacterial, viral, fungal, and parasitic agents. Noninfectious inflammatory processes include surgery, intrathecal hemorrhage, and the administration of intrathecal (inside the dural canal) agents such as myelographic contrast media, anesthetics, and steroids.
Neoplasia includes the hematogenous spread of systemic tumors, such as breast and lung carcinoma, melanoma, and non-Hodgkin lymphoma. Neoplasia also includes direct seeding of the cerebrospinal fluid (CSF) from primary central nervous system (CNS) tumors such as glioblastoma multiforme, medulloblastoma, ependymoma, and choroid plexus carcinoma. Strictly speaking, the most common cause of arachnoiditis in failed back syndrome is not infectious or from cancer. It is due to non-specific scarring secondary to the surgery or the underlying pathology.[116] [117] [118] [119] [120] [121] [122] [123]
Nerve injury
Laceration of a nerve root, or damage from cautery or traction can lead to chronic pain, however this can be difficult to determine. Chronic compression of the nerve root by a persistent agent such as disc, bone (osteophyte) or scarring can also permanently damage the nerve root. Epidural scarring caused by the initial pathology or occurring after the surgery can also contribute to nerve damage. In one study of failed back patients, the presence of pathology was noted to be at the same site as the level of surgery performed in 57% of cases. The remaining cases developed pathology at a different level, or on the opposite side, but at the same level as the surgery was performed. In theory, all failed back patients have some sort of nerve injury or damage which leads to a persistence of symptoms after a reasonable healing time. [124] [125] [126]
Return to work
In a groundbreaking Canadian study, Waddell et al. [127] reported on the value of repeat surgery and the return to work in worker’s compensation cases. They concluded that workers who undergo spinal surgery take longer to return to their jobs. Once two spinal surgeries are performed, few if any ever return to gainful employment of any kind. After two spinal surgeries, most people in the worker’s comp system will not be made better by more surgery. Most will be worse after a third surgery.
Episodes of back pain associated with on the job injuries in the worker’s compensation setting are usually of short duration. About 10% of such episodes will not be simple, and will degenerate into chronic and disabling back pain conditions, even if surgery is not performed. [128] [129]
It has been hypothesized that job dissatisfaction and individual perception of physical demands are associated with an increased time of recovery or an increased risk of no recovery at all. [130] Individual psychological and social work factors, as well as worker-employer relations are also likely to be associated with time and rates of recovery. [131] [132] [133]
A Finnish study of return to work in patients with spinal stenosis treated by surgery found that: (1) none of the patients who had retired before the operation returned to work afterward. (2) The variables that predicted postoperative ability to work for women were: being fit to work at the time of operation, age < 50 years at the time of operation, and duration of lumbar spinal stenosis symptoms < 2 years. (3) For men, these variables were: being fit to work at the time of operation, age < 50 years at the time of operation, no prior surgery, and the extent of the surgical procedure equal to or less than one laminectomy. Women's and men's working capacity do not differ after lumbar spinal stenosis operation. If the aim is to maximize working capacity, then, when a lumbar spinal stenosis operation is indicated, it should be performed without delay. In lumbar spinal stenosis patients who are > 50 years old and on sick leave, it is unrealistic to expect that they will return to work. Therefore, after such an extensive surgical procedure, re-education of patients for lighter jobs could improve the chances of these patients returning to work. [134]
In a related Finnish study, a total of 439 patients operated on for lumbar spinal stenosis during the period 1974-1987 was re-examined and evaluated for working and functional capacity approximately 4 years after the decompressive surgery. The ability to work before or after the operation and a history of no prior back surgery were variables predictive of a good outcome. Before the operation 86 patients were working, 223 patients were on sick leave, and 130 patients were retired. After the operation 52 of the employed patients and 70 of the unemployed patients returned to work. None of the retired patients returned to work. Ability to work preoperatively, age under 50 years at the time of operation and the absence of prior back surgery predicted a postoperative ability to work. [135]
Template:Skin and subcutaneous tissue symptoms and signs Template:Nervous and musculoskeletal system symptoms and signs Template:Urinary system symptoms and signs Template:Cognition, perception, emotional state and behaviour symptoms and signs Template:Speech and voice symptoms and signs Template:General symptoms and signs
- ↑ Long DM (1991). "Failed back surgery syndrome". Neurosurg Clin N Am. 2 (4): 899–919. PMID 1840393. Unknown parameter
|month=ignored (help) - ↑ Fritsch EW, Heisel J, Rupp S (1996). "The failed back surgery syndrome: reasons, intraoperative findings, and long-term results: a report of 182 operative treatments". Spine. 21 (5): 626–33. doi:10.1097/00007632-199603010-00017. PMID 8852320. Unknown parameter
|month=ignored (help) - ↑ Slipman CW, Shin CH, Patel RK; et al. (2002). "Etiologies of failed back surgery syndrome". Pain Med. 3 (3): 200–14, discussion 214–7. doi:10.1046/j.1526-4637.2002.02033.x. PMID 15099254. Unknown parameter
|month=ignored (help) - ↑ Taylor VM, Deyo RA, Cherkin DC, Kreuter W (1994). "Low back pain hospitalization. Recent United States trends and regional variations". Spine. 19 (11): 1207–12, discussion 13. PMID 8073311. Unknown parameter
|month=ignored (help) - ↑ Fager, C. A., Freiberg, S. R., Spine, 5:87-94; 1980
- ↑ Spengler, D. M., et. al. Spine 5:356-60; 1980
- ↑ Wiltse, l. L., Rocchio, P. D., J. Bone Joint Surg.; 57 A:478-83, 1957
- ↑ Burton, C. V., et. al., Clin. Orthop. 157:191-99; 1981
- ↑ Hakelius, A., Acta. Orthop. Scand., Suppl:129-76; 1970
- ↑ Turner, J., et al., Spine 1992; 17:1-8
- ↑ Mardjetko, S. M., et al., Spine 1994; 20S:2256S-2265S
- ↑ Herron, L. D., and Trippi, A. C., Spine 1989; 14:534-538
- ↑ Epstein, N. E., J. Spinal Disorder. 1998; 11(2): 116-122
- ↑ Deyo RA, Gray DT, Kreuter W, Mirza S, Martin BI (2005). "United States trends in lumbar fusion surgery for degenerative conditions". Spine. 30 (12): 1441–5, discussion 1446–7. doi:10.1097/01.brs.0000166503.37969.8a. PMID 15959375. Unknown parameter
|month=ignored (help) - ↑ 15.0 15.1 Abelson, R and Petersen, M. “An operation to ease back pain bolsters the bottom line, too.” New York Times, December 31, 2003.
- ↑ Abelson, R. “Surgeons invest in makers of hardware”. New York Times, December 30, 2006.
- ↑ Guyer RD, Patterson M, Ohnmeiss DD (2006). "Failed back surgery syndrome: diagnostic evaluation". J Am Acad Orthop Surg. 14 (9): 534–43. PMID 16959891. Unknown parameter
|month=ignored (help) - ↑ 18.0 18.1 18.2 Deyo RA (2002). "Diagnostic evaluation of LBP: reaching a specific diagnosis is often impossible". Arch Intern Med. 162 (13): 1444–7, discussion 1447–8. doi:10.1001/archinte.162.13.1444. PMID 12090877. Unknown parameter
|month=ignored (help) - ↑ 19.0 19.1 Carragee EJ (2005). "Clinical practice. Persistent low back pain". N Engl J Med. 352 (18): 1891–8. doi:10.1056/NEJMcp042054. PMID 15872204. Unknown parameter
|month=ignored (help) - ↑ 20.0 20.1 Levin DA, Hale JJ, Bendo JA (2007). "Adjacent segment degeneration following spinal fusion for degenerative disc disease". Bull NYU Hosp Jt Dis. 65 (1): 29–36. PMID 17539759.
- ↑ 21.0 21.1 21.2 Peng B, Wu W, Li Z, Guo J, Wang X (2007). "Chemical radiculitis". Pain. 127 (1–2): 11–6. doi:10.1016/j.pain.2006.06.034. PMID 16963186. Unknown parameter
|month=ignored (help) - ↑ Marshall LL, Trethewie ER (1973). "Chemical irritation of nerve-root in disc prolapse". Lancet. 2 (7824): 320. doi:10.1016/S0140-6736(73)90818-0. PMID 4124797. Unknown parameter
|month=ignored (help) - ↑ McCarron RF, Wimpee MW, Hudkins PG, Laros GS (1987). "The inflammatory effect of nucleus pulposus. A possible element in the pathogenesis of low-back pain". Spine. 12 (8): 760–4. doi:10.1097/00007632-198710000-00009. PMID 2961088. Unknown parameter
|month=ignored (help) - ↑ Takahashi H, Suguro T, Okazima Y, Motegi M, Okada Y, Kakiuchi T (1996). "Inflammatory cytokines in the herniated disc of the lumbar spine". Spine. 21 (2): 218–24. doi:10.1097/00007632-199601150-00011. PMID 8720407. Unknown parameter
|month=ignored (help) - ↑ Igarashi T, Kikuchi S, Shubayev V, Myers RR (2000). "2000 Volvo Award winner in basic science studies: Exogenous tumor necrosis factor-alpha mimics nucleus pulposus-induced neuropathology. Molecular, histologic, and behavioral comparisons in rats". Spine. 25 (23): 2975–80. doi:10.1097/00007632-200012010-00003. PMID 11145807. Unknown parameter
|month=ignored (help) - ↑ Sommer C, Schafers M (2004). "Mechanisms of neuropathic pain: the role of cytokines". Drug Discovery Today: Disease Mechanisms. 1 (4): 441–8. doi:10.1016/j.ddmec.2004.11.018. Unknown parameter
|month=ignored (help) - ↑ Igarashi A, Kikuchi S, Konno S, Olmarker K (2004). "Inflammatory cytokines released from the facet joint tissue in degenerative lumbar spinal disorders". Spine. 29 (19): 2091–5. doi:10.1097/01.brs.0000141265.55411.30. PMID 15454697. Unknown parameter
|month=ignored (help) - ↑ Sakuma Y, Ohtori S, Miyagi M; et al. (2007). "Up-regulation of p55 TNF alpha-receptor in dorsal root ganglia neurons following lumbar facet joint injury in rats". Eur Spine J. 16 (8): 1273–8. doi:10.1007/s00586-007-0365-3. PMC 2200776. PMID 17468886. Unknown parameter
|month=ignored (help) - ↑ Sekiguchi M, Kikuchi S, Myers RR (2004). "Experimental spinal stenosis: relationship between degree of cauda equina compression, neuropathology, and pain". Spine. 29 (10): 1105–11. doi:10.1097/00007632-200405150-00011. PMID 15131438. Unknown parameter
|month=ignored (help) - ↑ Séguin CA, Pilliar RM, Roughley PJ, Kandel RA (2005). "Tumor necrosis factor-alpha modulates matrix production and catabolism in nucleus pulposus tissue". Spine. 30 (17): 1940–8. doi:10.1097/01.brs.0000176188.40263.f9. PMID 16135983. Unknown parameter
|month=ignored (help) - ↑ Spengler, D. M., et al. Spine 5:356-60, 1980
- ↑ Wiltse, L. L., Rocchio, P. D., J. Bone Joint Surg., 57A:478-83, 1980
- ↑ Weir, B. K. A., J. Neuro. Surg. 50:283-89, 1979
- ↑ Weir, B. K. A., Jacobs, G. A., Spine 5:366-70, 1980
- ↑ Burton, C. V., et al. Clin Orthop. 157:191-99, 1981
- ↑ Oaklnader, A. L., and North, R. B. “Failed back surgery syndrome” In Loeser, J. D., et al., eds. “Bonica’s Management of Pain”, Philadephia, Lippincott Williams & Williams, 2001
- ↑ Haider, T T., et al., J. Occup. Rehabil. 8:247-253, 1998
- ↑ Tandon. V., Spine 24:1833-1838, 1999
- ↑ Turner, J., et al., JAMA 268:907-911, 1992
- ↑ Malter, A. D., et al., Spine 21:1048-1055, 1996
- ↑ Dvorak, J., et al., Spine 13:1418-1422, 1988
- ↑ Deyo, R., et al., J. Bone Joint Surg., 74 A:536-543, 1992
- ↑ Gervitz, R. N., et al., Prof. Psychol. Res. Pract. 27:561-566, 1996
- ↑ Graver, V., et al., Br. J. Neurosurg. 2:178-184, 1999
- ↑ de Groot, K. I., et al., Pain 69:19-25, 1997
- ↑ Schade, V., et al., Pain 80:239-249, 1999
- ↑ Rosenstiel, A., Keefe, F., Pain 17:33-40, 1983
- ↑ Keane GP (1997). "Failed low back surgery syndrome". In Herring SA, Cole AJ. The low back pain handbook: a practical guide for the primary care clinician. Philadelphia: Hanley & Belfus. pp. 269–81. ISBN 1-56053-152-5.
- ↑ Chatterjee S, Foy PM, Findlay GF (1995). "Report of a controlled clinical trial comparing automated percutaneous lumbar discectomy and microdiscectomy in the treatment of contained lumbar disc herniation". Spine. 20 (6): 734–8. doi:10.1097/00007632-199503150-00016. PMID 7604351. Unknown parameter
|month=ignored (help) - ↑ Linton, R. R., White, P. D., Arch. Surg., 50:6, 1945
- ↑ Epps, C. H., “Complications in Orthopedic Surgery”, p. 1009-1037, Lippincott and Co., Philadelphia, 1978
- ↑ Cauchoix, J., et al. Spine 3:256-59, 1978
- ↑ Weir, BKA., Jacobs, G. A., Spine ibid
- ↑ Benoist, M., et al., Spine 5:432-35, 1980
- ↑ Benner, B., Ehni, G., Spine 3: 40-44, 1978
- ↑ Rothman, R., Orhop. Clin. North Amer. 6:310, 1975
- ↑ Quiles, M., et al., Spine 3:45-5-, 1978
- ↑ Spangfort, E., Acta Orthop. Scand. Suppl 142:5-93, 1972
- ↑ Weir, B. K. A., Spine 5:366-70, 1980
- ↑ Benoist, M., et al., Spine 5:432-35, 1980
- ↑ Bener, B., Ehni, G., Spine 3:40-44, 1978
- ↑ Byrd, S. E., et al., Spine 10:652-61, 1985
- ↑ Deburge, A., Badelon, O., Rev. Chir. Orthop. 68:249-54, 1982 (French)
- ↑ Irstam, L., Spine 9:759-63, 1984
- ↑ Thibierge, M., Metzger, J., Rev. Chir. Orthop. 68:230-33, 1982 (French)
- ↑ Burton, C. V., et al., Clin. Orthop. 157:191-99, 1981
- ↑ Byrd, S. E., et al., Spine 10:652-61, 1985
- ↑ Massare, C., Rev. Chir. Orthop., 68:233-46, 1982
- ↑ Teplik, J. G., Haskin, M. E., Am. J. Neuroradiol., 43:845-55, 1984
- ↑
- ↑ Burton, C. V., et al., Clin Orthop. 157:191-99, 1981
- ↑ Crock, H. V., J. Bone Joint Surg. 58B:193-199, 1976
- ↑ Crock, H. V., “Practice of Spinal Surgery”, Vienna/New York; Springer Verlag, 1983
- ↑ Atlas, S. J., et al. Spine30:857-9, 2005
- ↑ Herno, A., Ann. Chir. Gynaecol. Suppl. 210:1-969, 1995
- ↑ Herno, A., et al. Spine 24:33-7, 1999
- ↑ Herno, A., et al., Spine 24:1010-4, 1999
- ↑ Herno, A., et al. Spine 24:2234-9, 1999
- ↑ Caputy, A. J., Luessenhop, A. J., J. Neurosurg. 77:669-76, 1992
- ↑ Sponseller, P. D., et al., Spine 25:2461-2466:2000
- ↑ Weinstein, M. A., et al., J. Spinal Disord. 13:422-426, 2000
- ↑ Massie, J. B., et al., Clin. Orthop. Rel. Res. 284:99-108, 1992
- ↑ Rechtine, G. R., et al., J. Ortho. Trauma 15:566-569, 2001
- ↑ Eck, K. R., et al., Spine 26::E182-E191, 201
- ↑ Capen, D. A., et al., Orthop. Clin. North. Am. 27:83-86, 1996
- ↑ Hee, H. T., et al., J. Spinal Disord. 14:533-540, 2001
- ↑ Aydinli, U., et al., Acta Orthop. Belg. 65:182-187, 1999
- ↑ Wimmer, C., et al. J. Spinal Disord. 11:498-500, 1998
- ↑ Wimmer, C., et al., J. Spinal Disoder. 11:124-128, 1998
- ↑ Hodges, S. D., et al., South. Med. J. 91:1132-1135, 1998
- ↑ Perry, J. W., et al., Clin. Infect. Dis. 24:558-561, 1997
- ↑ Abbey, D. M., et al., J. Spinal. Disord. 8:278-283, 1995
- ↑ West, J. L., et al., Spine 16:576-579, 1991
- ↑ Esses, S. I., et al., Spine 18:2238-2239, 1993
- ↑ Dave, S. H., and Meyers, D. L., Spine 17:(6 Suppl): S184-189, 1992
- ↑ Andreshak, T. G., et al., J. Spinal Disord. 10:376-379, 1997
- ↑ Viola, R. W., et al., Spine 22:2450-2451, 1997
- ↑ Klein, J. D., et al., Spine 22:2676-2682, 1996
- ↑ Swank, S. M., et al., J. Bone Joint Surg., 63 A: S268, 1981
- ↑ Klein, J. D., Garfin, S. R., Orthop. Clin. North. Am. 27:33-36, 1996
- ↑ Heary, R. F., et al., Surg. Neurol. 42:417-423, 1994
- ↑ Benoist, M., et al., Spine 5:432-35, 1980
- ↑ Langenskold, A., Valle, M., Clin. Orthop. 115:92-95, 1976
- ↑ La Rocca, H., Macnab, I., J. Bone Joint Surgery 56B:545-50, 1974
- ↑ Law, J. D., et al. J. Neurosurgery 48:259-63, 1978
- ↑ Lee, C. K., Alexander, H., Spine 9:305-12, 1984
- ↑ Lehmann, T. R., La Rocca, H., Spine 6:615-19,1981
- ↑ Lahde, S., Puranen, J., Eur. J. Radiol. 5:190-192, 1985
- ↑ Hinton, J. L. Jar, Wreck, D. J., Spine 20:564-570, 1995
- ↑ Fischgrund, J. S., J. Am. Acad. Orthop. Surg. 8:339-343, 2000
- ↑ Benoit, M., et al., Spine 5:432-35, 1980
- ↑ Benner, B., Ehni, G., Spine 3:40-44, 1978
- ↑ Brodsky, A. E., Spine 3:88-91, 1978
- ↑ Burton, C. V., Spine 3:24-30, 1978
- ↑ Quiles, M., et al., Spine 3:45-50, 1978
- ↑ Brammah, T. B., Jayson, M. I.,Spine 19(22):2603-5, 1994
- ↑ Georgy, B. A., et al., AJR Am. J. Roentgenol., 166(1):173-9, 1996
- ↑ Gero, B., et al., AJNR Am. J. Neuroradiol., 12(5):1009-19, 1991
- ↑ Gupta, R. K., et al., Neuroradiology 36:(1):39-43, 1994
- ↑ Johnson, C. E., Sze, G., AJNR Am. J. Neuroradiol. 11(4):763070, 1990
- ↑ Munoz, A., et al., AJNR 28:889-894, 2007
- ↑ Sharma, A., et al., AJR Am. J. Roentgenol. 168:807-12, 1997
- ↑ Tali. E. T., et al., Invest. Radiol. 37:152-9, 2002
- ↑ Chacoix, J., et al., Spine 3:256-59, 1978
- ↑ Weir, B.K.A., Jacobs, G. A., Spine 5:366-70, 1980
- ↑ Yong, H. K., et al., Spine 5:59-64, 1980
- ↑ Waddell, G., et al. J. Bone Joint Surgery, 61 A, 201-206, 1979
- ↑ Waddell, G., “The Back Pain Revolution”, London: Churchill Livingstone; 1998
- ↑ Litton, S., van Tulder, M., Spine 26:339-44, 2001
- ↑ Mielenz, T. J., et al., Spine 33:1270-1275, 2008
- ↑ Hoogendoorn, W. E., et al., Spine 25:2114-25, 2000
- ↑ Davis, K., Heaney, C., Clin. Biomech. 15:389-406, 2000
- ↑ Linton, S., et al., J. Occup. Rehab., 4:1-10, 1994
- ↑ Herno, A., et al., Am. J. Ind. Med. 30:473-8, 1996
- ↑ Airaksinen, O., et al., Eur Spine J. 3:261-4, 1994