Cytochrome c: Difference between revisions
No edit summary |
m (Bot: Automated text replacement (-{{SIB}} + & -{{EH}} + & -{{EJ}} + & -{{Editor Help}} + & -{{Editor Join}} +)) |
||
| Line 43: | Line 43: | ||
}} | }} | ||
{{SI}} | {{SI}} | ||
==Overview== | ==Overview== | ||
| Line 124: | Line 124: | ||
{{Electron transport chain}} | {{Electron transport chain}} | ||
[[Category:Cellular respiration]] | [[Category:Cellular respiration]] | ||
Revision as of 00:25, 9 August 2012
| Cytochrome c, somatic | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
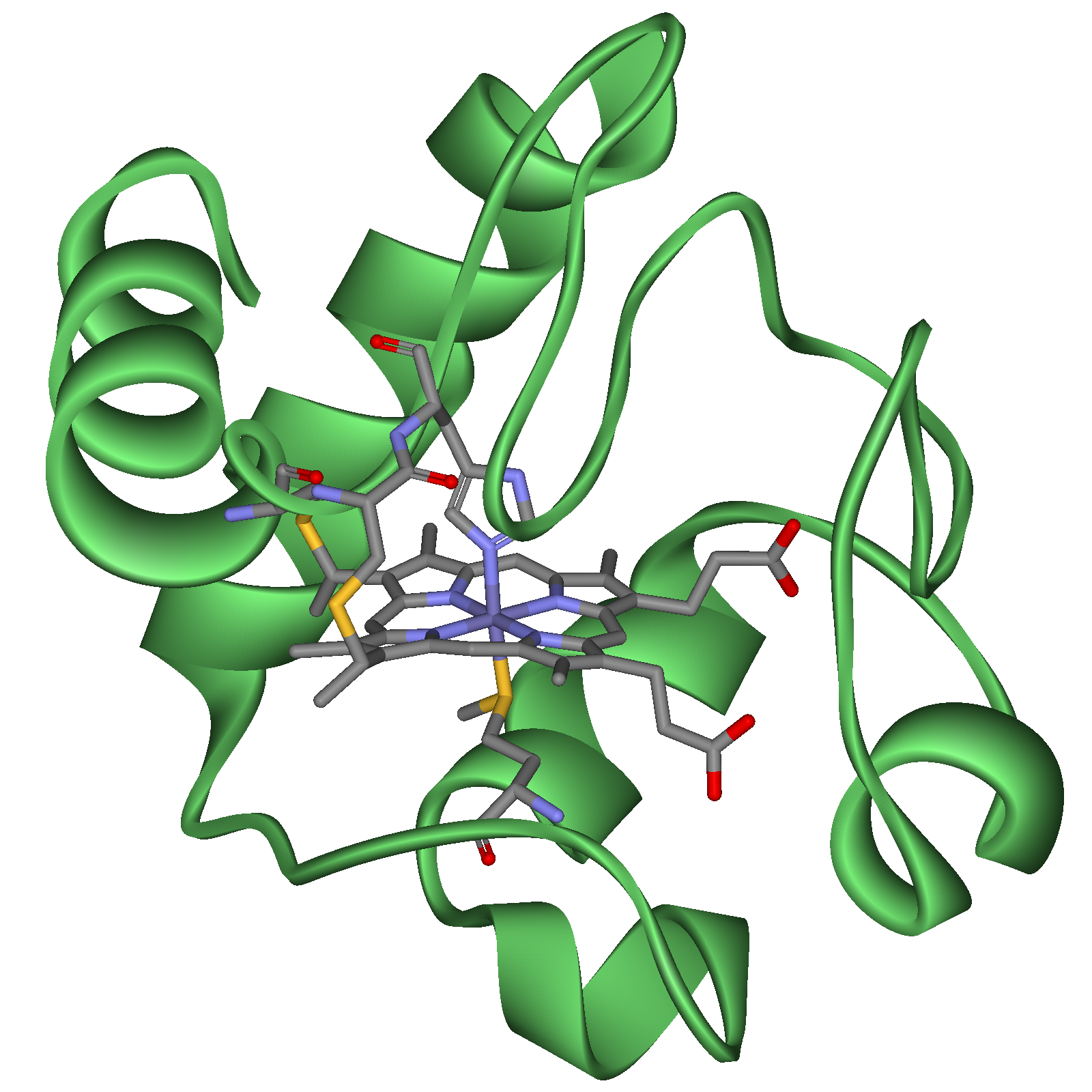 Cytochrome c with heme | |||||||||||
| Identifiers | |||||||||||
| Symbols | CYCS ; HCS; CYC | ||||||||||
| External IDs | Template:OMIM5 Template:MGI HomoloGene: 68675 | ||||||||||
| |||||||||||
| RNA expression pattern | |||||||||||
 | |||||||||||
| More reference expression data | |||||||||||
| Orthologs | |||||||||||
| Template:GNF Ortholog box | |||||||||||
| Species | Human | Mouse | |||||||||
| Entrez | n/a | n/a | |||||||||
| Ensembl | n/a | n/a | |||||||||
| UniProt | n/a | n/a | |||||||||
| RefSeq (mRNA) | n/a | n/a | |||||||||
| RefSeq (protein) | n/a | n/a | |||||||||
| Location (UCSC) | n/a | n/a | |||||||||
| PubMed search | n/a | n/a | |||||||||
|
WikiDoc Resources for Cytochrome c |
|
Articles |
|---|
|
Most recent articles on Cytochrome c Most cited articles on Cytochrome c |
|
Media |
|
Powerpoint slides on Cytochrome c |
|
Evidence Based Medicine |
|
Clinical Trials |
|
Ongoing Trials on Cytochrome c at Clinical Trials.gov Clinical Trials on Cytochrome c at Google
|
|
Guidelines / Policies / Govt |
|
US National Guidelines Clearinghouse on Cytochrome c
|
|
Books |
|
News |
|
Commentary |
|
Definitions |
|
Patient Resources / Community |
|
Patient resources on Cytochrome c Discussion groups on Cytochrome c Patient Handouts on Cytochrome c Directions to Hospitals Treating Cytochrome c Risk calculators and risk factors for Cytochrome c
|
|
Healthcare Provider Resources |
|
Causes & Risk Factors for Cytochrome c |
|
Continuing Medical Education (CME) |
|
International |
|
|
|
Business |
|
Experimental / Informatics |
Overview
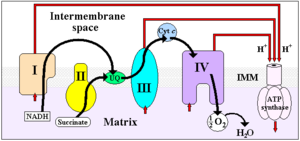
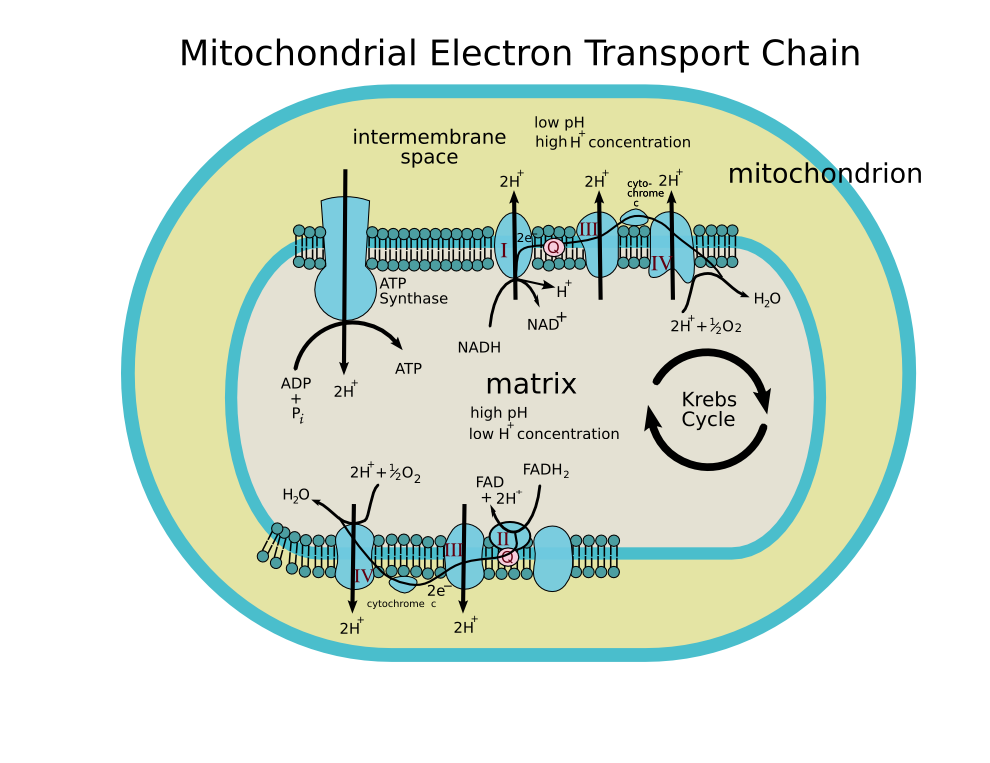
Cytochrome c, or cyt c (horse heart: PDB 1HRC) is a small heme protein found loosely associated with the inner membrane of the mitochondrion. It is a soluble protein, unlike other cytochromes, and is an essential component of the electron transfer chain, where it carries one electron. It is capable of undergoing oxidation and reduction, but does not bind oxygen. It transfers electrons between Complexes III and IV. It belongs to cytochrome c family of proteins.
Variation

Cytochrome c is a highly conserved protein across the spectrum of species, found in plants, animals, and many unicellular organisms. This, along with its small size (molecular weight about 12,000 daltons), makes it useful in studies of cladistics. Its primary structure consists of a chain of 100 amino acids.
The cytochrome c molecule has been studied for the glimpse it gives into evolutionary biology. Both chickens and turkeys have the identical molecule (amino acid for amino acid) within their mitochondria, whereas ducks possess molecules differing by one amino acid. Similarly, both humans and chimpanzees have the identical molecule, while rhesus monkeys possess cytochromes differing by one amino acid.
Functions
Cytochrome c can catalyze several reactions such as hydroxylation and aromatic oxidation, and shows peroxidase activity by oxidation of various electron donors such as 2,2-azino-bis(3-ethylbenzthiazoline-6-sulphonic acid) (ABTS), 2-keto-4-thiomethyl butyric acid and 4-aminoantipyrine.
Role in low level laser therapy
Cytochrome c is also suspected to be the functional complex in so called LLLT: Low-level laser therapy. In LLLT, laser light on the wavelength of 670 nanometer penetrates wounded and scarred tissue in order to increase cellular regeneration. Light of this wavelength appears capable of increasing activity of cytochrome c, thus increasing metabolic activity and freeing up more energy for the cells to repair the tissue.[citation needed]
Role in apoptosis
Cytochrome c is also an intermediate in apoptosis, a controlled form of cell death used to kill cells in the process of development or in response to infection or DNA damage[1] .
Cytochrome c is released by the mitochondria in response to pro-apoptotic stimuli. The sustained elevation in calcium levels precedes cyt c release from the mitochondria. The release of small amounts of cyt c leads to an interaction with the IP3 receptor (IP3R) on the endoplasmic reticulum (ER), causing ER calcium release. The overall increase in calcium triggers a massive release of cyt c, which then acts in the positive feedback loop to maintain ER calcium release through the IP3Rs. This explains how the ER calcium release can reach cytotoxic levels. This release in turn activates caspase 9, a cysteine protease. Caspase 9 can then go on to activate caspases 3 and 7, which are responsible for destroying the cell from within.
Classes
In 1991 R. P. Ambler recognized four classes of cytochrome c:
- Class I includes the lowspin soluble cytochrome c of mitochondria and bacteria. It has the heme-attachment site towards the N terminus of histidine and the sixth ligand provided by a methionine residue towards the C terminus.
- Class II includes the highspin cytochrome c'. It has the heme-mattachment site closed to the N terminus of histidine.
- Class III comprises the low redox potential multiple heme cytochromes. The heme c groups are structurally and functionally nonequivalent and present different redox potentials in the range 0 to -400 mV.
- Class IV was originally created to hold the complex proteins that have other prosthetic groups as well as heme c.
References
Further reading
- Skulachev VP (1998). "Cytochrome c in the apoptotic and antioxidant cascades". FEBS Lett. 423 (3): 275–80. PMID 9515723.
- Mannella CA (1998). "Conformational changes in the mitochondrial channel protein, VDAC, and their functional implications". J. Struct. Biol. 121 (2): 207–18. doi:10.1006/jsbi.1997.3954. PMID 9615439.
- Ferri KF, Jacotot E, Blanco J; et al. (2001). "Mitochondrial control of cell death induced by HIV-1-encoded proteins". Ann. N. Y. Acad. Sci. 926: 149–64. PMID 11193032.
- Britton RS, Leicester KL, Bacon BR (2002). "Iron toxicity and chelation therapy". Int. J. Hematol. 76 (3): 219–28. PMID 12416732.
- Haider N, Narula N, Narula J (2003). "Apoptosis in heart failure represents programmed cell survival, not death, of cardiomyocytes and likelihood of reverse remodeling". J. Card. Fail. 8 (6 Suppl): S512–7. doi:10.1054/jcaf.2002.130034. PMID 12555167.
- Castedo M, Perfettini JL, Andreau K; et al. (2004). "Mitochondrial apoptosis induced by the HIV-1 envelope". Ann. N. Y. Acad. Sci. 1010: 19–28. PMID 15033690.
- Ng S, Smith MB, Smith HT, Millett F (1977). "Effect of modification of individual cytochrome c lysines on the reaction with cytochrome b5". Biochemistry. 16 (23): 4975–8. PMID 199233.
- Lynch SR, Sherman D, Copeland RA (1992). "Cytochrome c binding affects the conformation of cytochrome a in cytochrome c oxidase". J. Biol. Chem. 267 (1): 298–302. PMID 1309738.
- Garber EA, Margoliash E (1990). "Interaction of cytochrome c with cytochrome c oxidase: an understanding of the high- to low-affinity transition". Biochim. Biophys. Acta. 1015 (2): 279–87. PMID 2153405.
- Bedetti CD (1985). "Immunocytochemical demonstration of cytochrome c oxidase with an immunoperoxidase method: a specific stain for mitochondria in formalin-fixed and paraffin-embedded human tissues". J. Histochem. Cytochem. 33 (5): 446–52. PMID 2580882.
- Tanaka Y, Ashikari T, Shibano Y; et al. (1988). "Construction of a human cytochrome c gene and its functional expression in Saccharomyces cerevisiae". J. Biochem. 103 (6): 954–61. PMID 2844747.
- Evans MJ, Scarpulla RC (1989). "The human somatic cytochrome c gene: two classes of processed pseudogenes demarcate a period of rapid molecular evolution". Proc. Natl. Acad. Sci. U.S.A. 85 (24): 9625–9. PMID 2849112.
- Passon PG, Hultquist DE (1972). "Soluble cytochrome b 5 reductase from human erythrocytes". Biochim. Biophys. Acta. 275 (1): 62–73. PMID 4403130.
- Dowe RJ, Vitello LB, Erman JE (1984). "Sedimentation equilibrium studies on the interaction between cytochrome c and cytochrome c peroxidase". Arch. Biochem. Biophys. 232 (2): 566–73. PMID 6087732.
- Michel B, Bosshard HR (1984). "Spectroscopic analysis of the interaction between cytochrome c and cytochrome c oxidase". J. Biol. Chem. 259 (16): 10085–91. PMID 6088481.
- Broger C, Nałecz MJ, Azzi A (1980). "Interaction of cytochrome c with cytochrome bc1 complex of the mitochondrial respiratory chain". Biochim. Biophys. Acta. 592 (3): 519–27. PMID 6251869.
- Smith HT, Ahmed AJ, Millett F (1981). "Electrostatic interaction of cytochrome c with cytochrome c1 and cytochrome oxidase". J. Biol. Chem. 256 (10): 4984–90. PMID 6262312.
- Geren LM, Millett F (1981). "Fluorescence energy transfer studies of the interaction between adrenodoxin and cytochrome c.". J. Biol. Chem. 256 (20): 10485–9. PMID 6270113.
- Favre B, Zolnierowicz S, Turowski P, Hemmings BA (1994). "The catalytic subunit of protein phosphatase 2A is carboxyl-methylated in vivo". J. Biol. Chem. 269 (23): 16311–7. PMID 8206937.
- Gao B, Eisenberg E, Greene L (1996). "Effect of constitutive 70-kDa heat shock protein polymerization on its interaction with protein substrate". J. Biol. Chem. 271 (28): 16792–7. PMID 8663341.
Additional images
-
ETC
-
ETC
See also
External links
- UMich Orientation of Proteins in Membranes families/superfamily-78 - Calculated orientations of cytochromes c in the lipid bilayer
- Cytochrome+c at the US National Library of Medicine Medical Subject Headings (MeSH)
- CS1 maint: Multiple names: authors list
- Human proteins
- All articles with unsourced statements
- Articles with unsourced statements from April 2008
- Articles with invalid date parameter in template
- CS1 maint: Explicit use of et al.
- Cellular respiration
- Cytochromes
- Programmed cell death
- Peripheral membrane proteins