Cyclin-dependent kinase 3
| Cyclin-dependent kinase 3 | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Identifiers | |||||||||
| Symbols | CDK3 ; | ||||||||
| External IDs | Template:OMIM5 HomoloGene: 74387 | ||||||||
| |||||||||
| RNA expression pattern | |||||||||
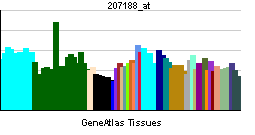 | |||||||||
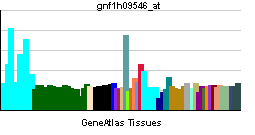 | |||||||||
| More reference expression data | |||||||||
| Orthologs | |||||||||
| Template:GNF Ortholog box | |||||||||
| Species | Human | Mouse | |||||||
| Entrez | n/a | n/a | |||||||
| Ensembl | n/a | n/a | |||||||
| UniProt | n/a | n/a | |||||||
| RefSeq (mRNA) | n/a | n/a | |||||||
| RefSeq (protein) | n/a | n/a | |||||||
| Location (UCSC) | n/a | n/a | |||||||
| PubMed search | n/a | n/a | |||||||
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]
Cyclin-dependent kinase 3, also known as CDK3, is a human gene.[1]
CDK3 complements cdc28 mutants of Saccharomyces cerevisiae suggesting that it may be involved in cell cycle control. CDK3 can phosphorylate histone H1 and interacts with an unknown type of cyclin.[1]
References
Further reading
- Meyerson M, Enders GH, Wu CL; et al. (1992). "A family of human cdc2-related protein kinases". EMBO J. 11 (8): 2909–17. PMID 1639063.
- Bullrich F, MacLachlan TK, Sang N; et al. (1995). "Chromosomal mapping of members of the cdc2 family of protein kinases, cdk3, cdk6, PISSLRE, and PITALRE, and a cdk inhibitor, p27Kip1, to regions involved in human cancer". Cancer Res. 55 (6): 1199–205. PMID 7882308.
- Gyuris J, Golemis E, Chertkov H, Brent R (1993). "Cdi1, a human G1 and S phase protein phosphatase that associates with Cdk2". Cell. 75 (4): 791–803. PMID 8242750.
- Sasaguri T, Ishida A, Kosaka C; et al. (1996). "Phorbol ester inhibits the phosphorylation of the retinoblastoma protein without suppressing cyclin D-associated kinase in vascular smooth muscle cells". J. Biol. Chem. 271 (14): 8345–51. PMID 8626531.
- Meikrantz W, Schlegel R (1996). "Suppression of apoptosis by dominant negative mutants of cyclin-dependent protein kinases". J. Biol. Chem. 271 (17): 10205–9. PMID 8626584.
- Hofmann F, Livingston DM (1996). "Differential effects of cdk2 and cdk3 on the control of pRb and E2F function during G1 exit". Genes Dev. 10 (7): 851–61. PMID 8846921.
- Lamphere L, Fiore F, Xu X; et al. (1997). "Interaction between Cdc37 and Cdk4 in human cells". Oncogene. 14 (16): 1999–2004. doi:10.1038/sj.onc.1201036. PMID 9150368.
- Braun K, Hölzl G, Soucek T; et al. (1998). "Investigation of the cell cycle regulation of cdk3-associated kinase activity and the role of cdk3 in proliferation and transformation". Oncogene. 17 (17): 2259–69. doi:10.1038/sj.onc.1202145. PMID 9811456.
- Yamochi T, Semba K, Tsuji K; et al. (2002). "ik3-1/Cables is a substrate for cyclin-dependent kinase 3 (cdk 3)". Eur. J. Biochem. 268 (23): 6076–82. PMID 11733001.
- Sato H, Nishimoto I, Matsuoka M (2002). "ik3-2, a relative to ik3-1/cables, is associated with cdk3, cdk5, and c-abl". Biochim. Biophys. Acta. 1574 (2): 157–63. PMID 11955625.
- Schang LM, Bantly A, Schaffer PA (2002). "Explant-induced reactivation of herpes simplex virus occurs in neurons expressing nuclear cdk2 and cdk4". J. Virol. 76 (15): 7724–35. PMID 12097586.
- Ota T, Suzuki Y, Nishikawa T; et al. (2004). "Complete sequencing and characterization of 21,243 full-length human cDNAs". Nat. Genet. 36 (1): 40–5. doi:10.1038/ng1285. PMID 14702039.
- Ren S, Rollins BJ (2004). "Cyclin C/cdk3 promotes Rb-dependent G0 exit". Cell. 117 (2): 239–51. PMID 15084261.
- Zhang Y, Wolf-Yadlin A, Ross PL; et al. (2005). "Time-resolved mass spectrometry of tyrosine phosphorylation sites in the epidermal growth factor receptor signaling network reveals dynamic modules". Mol. Cell Proteomics. 4 (9): 1240–50. doi:10.1074/mcp.M500089-MCP200. PMID 15951569.
- Beausoleil SA, Villén J, Gerber SA; et al. (2006). "A probability-based approach for high-throughput protein phosphorylation analysis and site localization". Nat. Biotechnol. 24 (10): 1285–92. doi:10.1038/nbt1240. PMID 16964243.
- Olsen JV, Blagoev B, Gnad F; et al. (2006). "Global, in vivo, and site-specific phosphorylation dynamics in signaling networks". Cell. 127 (3): 635–48. doi:10.1016/j.cell.2006.09.026. PMID 17081983.
- Wissing J, Jänsch L, Nimtz M; et al. (2007). "Proteomics analysis of protein kinases by target class-selective prefractionation and tandem mass spectrometry". Mol. Cell Proteomics. 6 (3): 537–47. doi:10.1074/mcp.T600062-MCP200. PMID 17192257.
External links
- CDK3+protein,+human at the US National Library of Medicine Medical Subject Headings (MeSH)
| Stub icon | This enzyme-related article is a stub. You can help Wikipedia by expanding it. |