Carcinoid syndrome pathophysiology: Difference between revisions
| Line 26: | Line 26: | ||
==Embryology== | ==Embryology== | ||
Carcinoid | Carcinoid tumors originate from [[neuroendocrine cells]] ([[enterochromaffin]] or amine precursor uptake and decarboxylase [APUD] cells), which embryologically are of neural crest origin. Gastrointestinal carcinoids derive from cells that migrate from the neural crest to the [[foregut]], [[midgut]] and [[hindgut]].<ref name="pmid17114072">{{cite journal| author=Reznek RH| title=CT/MRI of neuroendocrine tumours. | journal=Cancer Imaging | year= 2006 | volume= 6 | issue= | pages= S163-77 | pmid=17114072 | doi=10.1102/1470-7330.2006.9037 | pmc=PMC1805060 | url=http://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=17114072 }} </ref> | ||
==Associated Conditions== | ==Associated Conditions== | ||
Revision as of 18:36, 5 October 2015
|
Carcinoid syndrome Microchapters |
|
Diagnosis |
|---|
|
Treatment |
|
Case Studies |
|
Carcinoid syndrome pathophysiology On the Web |
|
American Roentgen Ray Society Images of Carcinoid syndrome pathophysiology |
|
Risk calculators and risk factors for Carcinoid syndrome pathophysiology |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [2]Associate Editor(s)-in-Chief: Parminder Dhingra, M.D. [3]
Overview
The pathophysiology of carcinoid tumor depends on the histological subtype. Genes involved in the pathogenesis of carcinoid tumor are β-catenin, NF1, and MEN1. Carcinoid tumors originate from neuroendocrine cells. On microscopic histopathological analysis, gastrointestinal carcinoid syndrome is characterized by solid or small trabecular clusters of neuroendocrine cells with uniform nuclei and abundant granular or faintly staining (clear) cytoplasm.
Pathogenesis
- When the carcinoid tumor is in the gastrointestinal tract, as it is in the great majority of cases, the serotonin and kallikrein are inactivated in the liver and manifestations of carcinoid syndrome do not occur until there are metastases to the liver.
- The flushing results from secretion of kallikrein, the enzyme that catalyzes the conversion of kininogen to lysyl-bradykinin. The latter is further converted to bradykinin, one of the most powerful vasodilators known.
- Large amounts of serotonin produces pellagra-like features including diarrhea.
- Carcinoid tumors arising in the bronchi reach the systemic circulation before passing through the liver and may be associated with bronchoconstriction and manifestations of carcinoid syndrome without liver metastases.
- There is thickening of mural and valvular endothelial surfaces of right-sided cardiac structures due to fibrosis. This is thought to occur as a response to circulating neuroendocrine substances (serotonin, bradykinin, histamine, prostaglandin etc) released into the blood stream by the original carcinoid tumor.[1]
- In the cecum, argentaffin-like EC-cell carcinoids are most common, become increasingly less common in the more distal colon, and are uncommon in the rectum. Most rectal carcinoids have L-cell differentiation.[2]
- Most jejunal and ileal carcinoids are argentaffin-positive, substance P–containing, and serotonin-producing EC-cell tumors that generate carcinoid syndrome when hepatic or retroperitoneal nodal metastases are present. L-cell, glucagon-like polypeptide-producing, and pancreatic polypeptide- and polypeptide YY-producing tumors occur less frequently.[2]
Genetics
- Gastrointestinal carcinoids occur in association with inherited syndromes, such as multiple endocrine neoplasia type 1 and neurofibromatosis type 1.[2]
- Multiple endocrine neoplasia type 1 is caused by alterations of the MEN1 gene located at chromosomal region 11q13. Most carcinoids associated with Multiple endocrine neoplasia type 1 appear to be of foregut origin.
- Neurofibromatosis type 1 is an autosomal dominant genetic disorder caused by alteration of the NF1 gene at chromosome 17q11. Carcinoids in patients with neurofibromatosis type 1 appear to arise primarily in the periampullary region.
- In sporadic gastrointestinal carcinoids, numerous chromosomal imbalances have been found by comparative genome hybridization analysis. Gains involving chromosomes 5, 14, 17 (especially 17q), and 19 and losses involving chromosomes 11 (especially 11q) and 18 appear to be the most common.
- The most frequently reported mutated gene in gastrointestinal carcinoids is β-catenin (CTNNB1).
Embryology
Carcinoid tumors originate from neuroendocrine cells (enterochromaffin or amine precursor uptake and decarboxylase [APUD] cells), which embryologically are of neural crest origin. Gastrointestinal carcinoids derive from cells that migrate from the neural crest to the foregut, midgut and hindgut.[3]
Associated Conditions
Goblet cell carcinoid is considered to be a hybrid between an exocrine and endocrine tumor derived from crypt cells of the appendix. They behave in a more aggressive manner than do classical appendiceal carcinoids. Spread is usually to regional lymph nodes, peritoneum, and particularly the ovary. They do not produce sufficient hormonal substances to cause the carcinoid or other endocrine syndromes. In fact, they more closely resemble exocrine than endocrine tumors. The term 'crypt cell carcinoma' has been used for them, and though perhaps more accurate than considering them carcinoids, has not been a successful competitor.
Location
Carcinoid tumors are normally found throughout the gastrointestinal tract from mouth to anus, with the highest concentration of cells in the appendix and small intestine. The pancreas contains a large number of these cells, the biliary tree only a few and the liver normally contains none. Fibrotic lesions are found on endocardium, particularly on the right side of the heart.
Gross Pathology
Gastrointestinal Carcinoid
In the gastric or intestinal wall, carcinoids may occur as firm white, yellow, or gray nodules and may be intramural masses or may protrude into the lumen as polypoid nodules. The overlying gastric or intestinal mucosa may be intact or have focal ulceration.
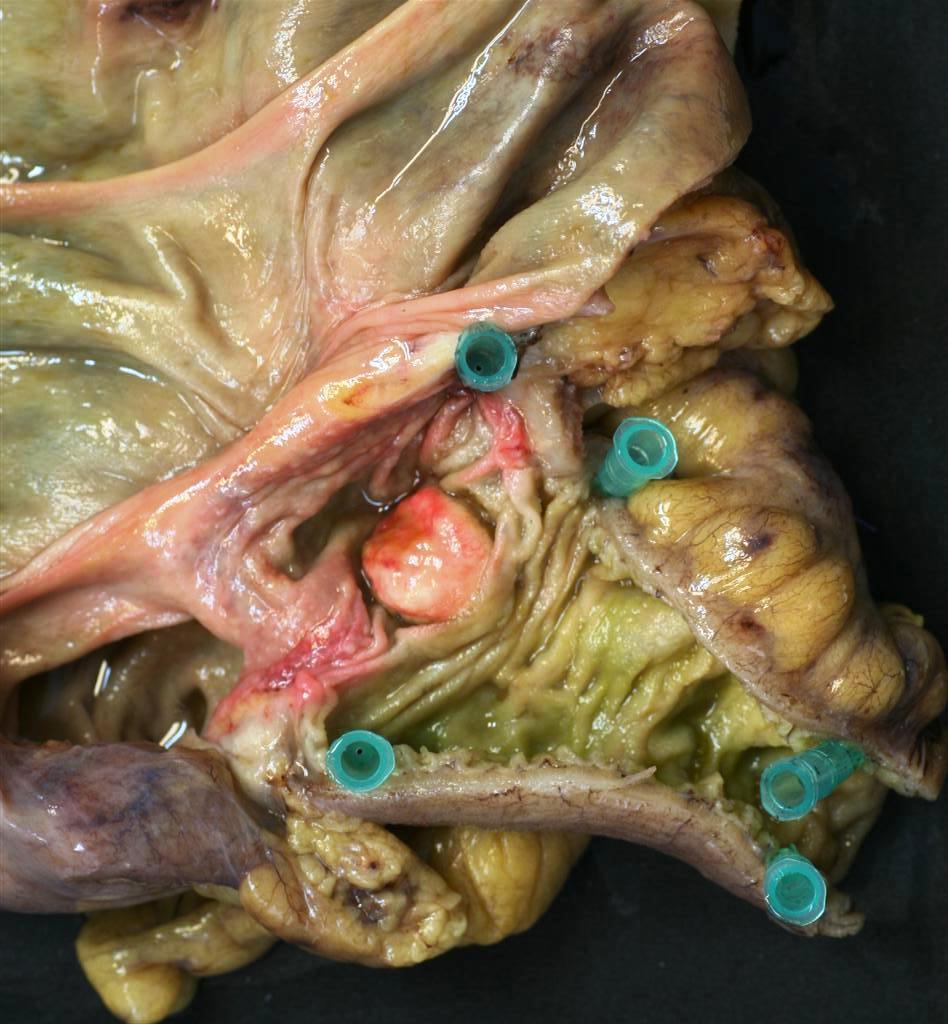 |
Ovarian Carcinoid
Lesions can markedly vary in size. Metastatic carcinoids are nearly always bilateral with scattered tumor deposits present throughout both ovaries. Primary carcinoids of the ovary are invariably unilateral. They form a solid nodule within a cystic teratoma, or form a pure solid hypervascular mass. They can be indistinguishable from other solid neoplasms of the ovary. Lesions can markedly vary in size. Metastatic carcinoids are nearly always bilateral with scattered tumor deposits present throughout both ovaries.[5]
Microscopic Pathology
Goblet Cell Carcinoid of Appendix
Histologically, globet cell carcinoid forms clusters of goblet cells containing mucin with a minor admixture of paneth cells and endocrine cells. The growth pattern is distinctive, typically producing a concentric band of tumor nests interspersed among the muscle and stroma of the appendiceal wall extending up the shaft of the appendix. This makes the lesion difficult to suspect grossly and difficult to measure. Small tumor nests may be camouflaged amongst the muscle or in periappendiceal fat, cytokeratin preparations best demonstrate the tumor cells, mucin stains are also helpful in identifying them.[6]
Gastric or Intestinal Carcinoid
Neuroendocrine cells have uniform nuclei and abundant granular or faintly staining (clear) cytoplasm, and are present as solid or small trabecular clusters, or are dispersed among other cells, which may make them difficult to recognize in sections stained with hematoxylin and eosin, immunostaining enables their exact identification. At the ultrastructural level, neuroendocrine cells contain cytoplasmic membrane-bound dense-cored secretory granules (diameter >80 nm) and may also contain small clear vesicles (diameter 40–80 nm) that correspond to the synaptic vesicles of neurons.[2]
Hepatic Carcinoid
Histologically, the tumor has features of classic carcinoid (i.e. trabecular and pseudoglandular pattern) and dense core granules demonstrated by electron microscopy or by immunohistochemistry (i.e. positive staining by chromogranin antibody).[7]
Lung Carcinoid
- Nests of cells
- Stippled chromatin
- Moderate cytoplasm
- No necrosis
- Low mitotic rate
-
Lung carcinoid low magnitude[8]
-
Lung carcinoid intermediate magnitude[8]
-
Lung carcinoid very high magnitude[8]
-
Lung carcinoid high magnitude[8]
Video
{{#ev:youtube|hTSHJBAD3C4}}
References
- ↑ Carcinoid cardiac lesions. Dr Henry Knipe and Dr Yuranga Weerakkody et al. Radiopaedia. http://radiopaedia.org/articles/carcinoid-cardiac-lesions
- ↑ 2.0 2.1 2.2 2.3 General Information About Gastrointestinal (GI) Carcinoid Tumors . National Cancer Institute. http://www.cancer.gov/types/gi-carcinoid-tumors/hp/gi-carcinoid-treatment-pdq#link/_49_toc Accessed on September 24, 2015
- ↑ Reznek RH (2006). "CT/MRI of neuroendocrine tumours". Cancer Imaging. 6: S163–77. doi:10.1102/1470-7330.2006.9037. PMC 1805060. PMID 17114072.
- ↑ Image courtesy of Dr Henry Knipe and Dr Yuranga Weerakkody et al. Radiopaedia (original file [1]). [http://radiopaedia.org/licence Creative Commons BY-SA-NC
- ↑ Ovarian carcinoid tumours. Dr Aditya Shetty and Dr Yuranga Weerakkody et al. Radiopaedia 2015. http://radiopaedia.org/articles/ovarian-carcinoid-tumours
- ↑ https://en.wikipedia.org/wiki/Carcinoid
- ↑ Hepatic carcinoid. Dr Henry Knipe and Dr Yuranga Weerakkody et al. Radiopaedia 2015. http://radiopaedia.org/articles/bronchial-carcinoid-tumour
- ↑ 8.0 8.1 8.2 8.3 Typical carcinoid lung tumour. Libre Pathology. http://librepathology.org/wiki/index.php/Typical_carcinoid_lung_tumour Accessed on September, 30 2015
![Lung carcinoid low magnitude[8]](/images/c/c0/Lung_carcinoid_low_mag.jpg)
![Lung carcinoid intermediate magnitude[8]](/images/c/c3/Lung_carcinoid_intermed_mag.jpg)
![Lung carcinoid very high magnitude[8]](/images/8/8a/Lung_carcinoid_very_high_mag.jpg)
![Lung carcinoid high magnitude[8]](/images/2/25/800px-Lung_carcinoid_-_high_mag.jpg)