Brimonidine (ophthalmic): Difference between revisions
Kiran Singh (talk | contribs) No edit summary |
Gerald Chi- (talk | contribs) mNo edit summary |
||
| (25 intermediate revisions by 2 users not shown) | |||
| Line 3: | Line 3: | ||
|genericName=Brimonidine | |genericName=Brimonidine | ||
|aOrAn=an | |aOrAn=an | ||
|drugClass= | |drugClass=alpha-2 adrenergic agonist | ||
|indicationType=treatment | |indicationType=treatment | ||
|indication= | |indication=[[open-angle glaucoma]] or [[ocular hypertension]] | ||
|adverseReactions=[[hypertension]], [[contact dermatitis]], [[erythema]], [[flushing]], sensation of burning of skin, [[xerostomia]], [[somnolence]], [[allergic conjunctivitis]], burning sensation in eye, conjunctival discoloration, conjunctival [[hyperemia]], follicular conjunctivitis, [[acute]] | |adverseReactions=[[hypertension]], [[contact dermatitis]], [[erythema]], [[flushing]], sensation of burning of skin, [[xerostomia]], [[somnolence]], [[allergic conjunctivitis]], burning sensation in eye, conjunctival discoloration, conjunctival [[hyperemia]], follicular [[conjunctivitis]], [[acute]] [[hypersensitivity reaction]], itching of eye, lid retraction, and [[visual disturbance]] | ||
|blackBoxWarningTitle=<b><span style="color:#FF0000;">TITLE</span></b> | |blackBoxWarningTitle=<b><span style="color:#FF0000;">TITLE</span></b> | ||
|blackBoxWarningBody=<i><span style="color:#FF0000;">Condition Name:</span></i> (Content) | |blackBoxWarningBody=<i><span style="color:#FF0000;">Condition Name:</span></i> (Content) | ||
|fdaLIADAdult=<h4> | |fdaLIADAdult=<h4>Ocular Hypertension</h4> | ||
* Dosing information | *Dosing information | ||
:* Recommended dose is one drop three times daily, approximately 8 hours apart. | :*Recommended dose is one drop three times daily, approximately 8 hours apart. Brimonidine ophthalmic solution may be used concomitantly with other topical ophthalmic drug products to lower [[intraocular pressure]]. If more than one topical ophthalmic product is to be used, the different products should be instilled at least 5 minutes apart. | ||
|offLabelAdultGuideSupport=There is limited information regarding <i>Off-Label Guideline-Supported Use</i> of | |offLabelAdultGuideSupport=There is limited information regarding <i>Off-Label Guideline-Supported Use</i> of brimonidine in adult patients. | ||
|offLabelAdultNoGuideSupport=There is limited information regarding <i>Off-Label Non–Guideline-Supported Use</i> of | |offLabelAdultNoGuideSupport=There is limited information regarding <i>Off-Label Non–Guideline-Supported Use</i> of brimonidine in adult patients. | ||
|offLabelPedGuideSupport=There is limited information regarding <i>Off-Label Guideline-Supported Use</i> of | |offLabelPedGuideSupport=There is limited information regarding <i>Off-Label Guideline-Supported Use</i> of brimonidine in pediatric patients. | ||
|offLabelPedNoGuideSupport=There is limited information regarding <i>Off-Label Non–Guideline-Supported Use</i> of | |offLabelPedNoGuideSupport=There is limited information regarding <i>Off-Label Non–Guideline-Supported Use</i> of brimonidine in pediatric patients. | ||
|contraindications=====Neonates and Infants (under the age of 2 years)==== | |contraindications=====Neonates and Infants (under the age of 2 years)==== | ||
*Brimonidine is contraindicated in neonates and infants (under the age of 2 years). | |||
====Hypersensitivity Reactions==== | ====Hypersensitivity Reactions==== | ||
*Brimonidine is contraindicated in patients who have exhibited a [[hypersensitivity]] reaction to any component of this medication in the past. | |||
|warnings=====Potentiation of Vascular Insufficiency==== | |warnings=====Potentiation of Vascular Insufficiency==== | ||
*Brimonidine may potentiate syndromes associated with vascular insufficiency. Brimonidine should be used with caution in patients with [[depression]], cerebral or coronary insufficiency, [[Raynaud's phenomenon]], [[orthostatic hypotension]], or [[thromboangiitis obliterans]]. | |||
====Severe Cardiovascular Disease==== | ====Severe Cardiovascular Disease==== | ||
Although brimonidine tartrate ophthalmic solution had minimal effect on the blood pressure of patients in clinical studies, caution should be exercised in treating patients with severe cardiovascular disease. | *Although brimonidine tartrate ophthalmic solution had minimal effect on the blood pressure of patients in clinical studies, caution should be exercised in treating patients with severe cardiovascular disease. | ||
====Contamination of Topical Ophthalmic Products After Use==== | ====Contamination of Topical Ophthalmic Products After Use==== | ||
There have been reports of bacterial [[keratitis]] associated with the use of multiple-dose containers of topical ophthalmic products. These containers had been inadvertently contaminated by patients who, in most cases, had a concurrent corneal disease or a disruption of the ocular epithelial surface. | *There have been reports of bacterial [[keratitis]] associated with the use of multiple-dose containers of topical ophthalmic products. These containers had been inadvertently contaminated by patients who, in most cases, had a concurrent corneal disease or a disruption of the ocular epithelial surface. | ||
|clinicalTrials=Because clinical studies are conducted under widely varying conditions, adverse reaction rates observed in the clinical studies of a drug cannot be directly compared to rates in the clinical studies of another drug and may not reflect the rates observed in practice. | |clinicalTrials=*Because clinical studies are conducted under widely varying conditions, adverse reaction rates observed in the clinical studies of a drug cannot be directly compared to rates in the clinical studies of another drug and may not reflect the rates observed in practice. | ||
Adverse reactions occurring in approximately 10-20% of the subjects receiving brimonidine ophthalmic solution (0.1-0.2%) included: [[allergic conjunctivitis]], conjunctival hyperemia, and eye [[pruritus]]. Adverse reactions occurring in approximately 5-9% included: burning sensation, conjunctival folliculosis, [[hypertension]], ocular allergic reaction, oral dryness, and [[visual disturbance]]. | *Adverse reactions occurring in approximately 10-20% of the subjects receiving brimonidine ophthalmic solution (0.1-0.2%) included: [[allergic conjunctivitis]], conjunctival hyperemia, and eye [[pruritus]]. Adverse reactions occurring in approximately 5-9% included: burning sensation, conjunctival folliculosis, [[hypertension]], ocular allergic reaction, oral dryness, and [[visual disturbance]]. | ||
Adverse reactions occurring in approximately 1-4% of the subjects receiving brimonidine ophthalmic solution (0.1-0.2%) included: abnormal taste, [[allergic reaction]], [[asthenia]], [[blepharitis]], blepharoconjunctivitis, blurred vision, [[bronchitis]], [[cataract]], conjunctival edema, [[conjunctival hemorrhage]], [[conjunctivitis]], [[cough]], [[dizziness]], [[dyspepsia]], [[dyspnea]], [[epiphora]], eye discharge, eye dryness, eye irritation, eye pain, eyelid edema, eyelid erythema, [[fatigue]], flu syndrome, follicular conjunctivitis, foreign body sensation, gastrointestinal disorder, [[headache]], [[hypercholesterolemia]], [[hypotension]], [[infection]] (primarily colds and respiratory infections), [[insomnia]], [[keratitis]], lid disorder, [[pharyngitis]], [[photophobia]], [[rash]], [[rhinitis]], [[sinus infection]], [[sinusitis]], [[somnolence]], stinging, superficial punctate keratopathy, tearing,visual field defect, vitreous detachment, vitreous disorder, vitreous floaters, and worsened visual acuity. | *Adverse reactions occurring in approximately 1-4% of the subjects receiving brimonidine ophthalmic solution (0.1-0.2%) included: abnormal taste, [[allergic reaction]], [[asthenia]], [[blepharitis]], blepharoconjunctivitis, [[blurred vision]], [[bronchitis]], [[cataract]], conjunctival edema, [[conjunctival hemorrhage]], [[conjunctivitis]], [[cough]], [[dizziness]], [[dyspepsia]], [[dyspnea]], [[epiphora]], [[eye discharge]], eye dryness, eye irritation, [[eye pain]], eyelid edema, eyelid erythema, [[fatigue]], flu syndrome, follicular conjunctivitis, foreign body sensation, gastrointestinal disorder, [[headache]], [[hypercholesterolemia]], [[hypotension]], [[infection]] (primarily colds and respiratory infections), [[insomnia]], [[keratitis]], lid disorder, [[pharyngitis]], [[photophobia]], [[rash]], [[rhinitis]], [[sinus infection]], [[sinusitis]], [[somnolence]], stinging, superficial punctate keratopathy, tearing, visual field defect, [[vitreous detachment]], vitreous disorder, vitreous floaters, and worsened [[visual acuity]]. | ||
The following reactions were reported in less than 1% of subjects: [[corneal erosion]], [[hordeolum]], nasal dryness, and taste perversion. | *The following reactions were reported in less than 1% of subjects: [[corneal erosion]], [[hordeolum]], nasal dryness, and taste perversion. | ||
|postmarketing=The following reactions have been identified during postmarketing use of brimonidine tartrate ophthalmic solutions in clinical practice. Because they are reported voluntarily from a population of unknown size, estimates of frequency cannot be made. The reactions, which have been chosen for inclusion due to either their seriousness, frequency of reporting, possible causal connection to brimonidine tartrate ophthalmic solutions, or a combination of these factors, include: [[bradycardia]], [[depression]], [[hypersensitivity]], [[iritis]], [[keratoconjunctivitis sicca]], [[miosis]], [[nausea]], skin reactions (including [[erythema]], eyelid [[pruritus]], [[rash]], and [[vasodilation]]), [[syncope]], and [[tachycardia]]. [[Apnea]], [[bradycardia]], [[coma]], [[hypotension]], [[hypothermia]], [[hypotonia]], [[lethargy]], [[pallor]], [[respiratory depression]], and [[somnolence]] have been reported in infants receiving brimonidine tartrate ophthalmic solutions. | |postmarketing=*The following reactions have been identified during postmarketing use of brimonidine tartrate ophthalmic solutions in clinical practice. Because they are reported voluntarily from a population of unknown size, estimates of frequency cannot be made. The reactions, which have been chosen for inclusion due to either their seriousness, frequency of reporting, possible causal connection to brimonidine tartrate ophthalmic solutions, or a combination of these factors, include: [[bradycardia]], [[depression]], [[hypersensitivity]], [[iritis]], [[keratoconjunctivitis sicca]], [[miosis]], [[nausea]], skin reactions (including [[erythema]], eyelid [[pruritus]], [[rash]], and [[vasodilation]]), [[syncope]], and [[tachycardia]]. [[Apnea]], [[bradycardia]], [[coma]], [[hypotension]], [[hypothermia]], [[hypotonia]], [[lethargy]], [[pallor]], [[respiratory depression]], and [[somnolence]] have been reported in infants receiving brimonidine tartrate ophthalmic solutions. | ||
|drugInteractions=====Antihypertensives/Cardiac Glycosides==== | |drugInteractions=====Antihypertensives/Cardiac Glycosides==== | ||
Because | *Because Brimonidine may reduce blood pressure, caution in using drugs such as [[antihypertensives]] and/or [[cardiac glycosides]] with Brimonidine is advised. | ||
====CNS Depressants==== | ====CNS Depressants==== | ||
Although specific drug interaction studies have not been conducted with | *Although specific drug interaction studies have not been conducted with Brimonidine, the possibility of an additive or potentiating effect with CNS depressants ([[alcohol]], [[barbiturates]], [[opiates]], [[sedatives]], or [[anesthetics]]) should be considered. | ||
====Tricyclic Antidepressants==== | ====Tricyclic Antidepressants==== | ||
[[Tricyclic antidepressants]] have been reported to blunt the hypotensive effect of systemic [[clonidine]]. It is not known whether the concurrent use of these agents with | *[[Tricyclic antidepressants]] have been reported to blunt the [[hypotensive]] effect of systemic [[clonidine]]. It is not known whether the concurrent use of these agents with Brimonidine in humans can lead to resulting interference with the [[IOP]] lowering effect. Caution is advised in patients taking [[tricyclic antidepressants]] which can affect the metabolism and uptake of circulating amines. | ||
====Monoamine Oxidase Inhibitors==== | ====Monoamine Oxidase Inhibitors==== | ||
Monoamine oxidase ([[MAO]]) inhibitors may theoretically interfere with the metabolism of brimonidine and potentially result in an increased systemic side-effect such as hypotension. Caution is advised in patients taking MAO inhibitors which can affect the metabolism and uptake of circulating amines. | *Monoamine oxidase ([[MAO]]) inhibitors may theoretically interfere with the metabolism of brimonidine and potentially result in an increased systemic side-effect such as [[hypotension]]. Caution is advised in patients taking MAO inhibitors which can affect the metabolism and uptake of circulating amines. | ||
|FDAPregCat=B | |FDAPregCat=B | ||
|useInPregnancyFDA= | |useInPregnancyFDA=*Teratogenicity studies have been performed in animals. | ||
Brimonidine tartrate was not teratogenic when given orally during gestation days 6 through 15 in rats and days 6 through 18 in rabbits. The highest doses of brimonidine tartrate in rats (2.5 mg/kg/day) and rabbits (5.0 mg/kg/day) achieved AUC exposure values 360- and 20-fold higher, or 260- and 15-fold higher, respectively, than similar values estimated in humans treated with | *Brimonidine tartrate was not teratogenic when given orally during gestation days 6 through 15 in rats and days 6 through 18 in rabbits. The highest doses of brimonidine tartrate in rats (2.5 mg/kg/day) and rabbits (5.0 mg/kg/day) achieved AUC exposure values 360- and 20-fold higher, or 260- and 15-fold higher, respectively, than similar values estimated in humans treated with Brimonidine 0.1% or 0.15%, 1 drop in both eyes three times daily. | ||
There are no adequate and well-controlled studies in pregnant women; however, in animal studies, brimonidine crossed the placenta and entered into the fetal circulation to a limited extent. Because animal reproduction studies are not always predictive of human response, | *There are no adequate and well-controlled studies in pregnant women; however, in animal studies, brimonidine crossed the [[placenta]] and entered into the fetal circulation to a limited extent. Because animal reproduction studies are not always predictive of human response, Brimonidine should be used during pregnancy only if the potential benefit to the mother justifies the potential risk to the fetus. | ||
|useInNursing=It is not known whether brimonidine tartrate is excreted in human milk, although in animal studies, brimonidine tartrate has been shown to be excreted in breast milk. Because of the potential for serious adverse reactions from | |useInNursing=*It is not known whether brimonidine tartrate is excreted in human milk, although in animal studies, brimonidine tartrate has been shown to be excreted in breast milk. Because of the potential for serious adverse reactions from Brimonidine in nursing infants, a decision should be made whether to discontinue nursing or to discontinue the drug, taking into account the importance of the drug to the mother. | ||
|useInPed= | |useInPed=*Brimonidine is contraindicated in children under the age of 2 years. During postmarketing surveillance, [[apnea]], [[bradycardia]], [[coma]], [[hypotension]], [[hypothermia]], [[hypotonia]], [[lethargy]], [[pallor]], [[respiratory depression]], and [[somnolence]] have been reported in infants receiving brimonidine. The safety and effectiveness of brimonidine tartrate have not been studied in children below the age of 2 years. | ||
In a well-controlled clinical study conducted in pediatric glaucoma patients (ages 2 to 7 years) the most commonly observed adverse reactions with brimonidine tartrate ophthalmic solution 0.2% dosed three times daily were somnolence (50-83% in patients ages 2 to 6 years) and decreased alertness. In pediatric patients 7 years of age (>20 kg), somnolence appears to occur less frequently (25%). Approximately 16% of patients on brimonidine tartrate ophthalmic solution discontinued from the study due to somnolence. | *In a well-controlled clinical study conducted in pediatric [[glaucoma]] patients (ages 2 to 7 years) the most commonly observed adverse reactions with brimonidine tartrate ophthalmic solution 0.2% dosed three times daily were [[somnolence]] (50-83% in patients ages 2 to 6 years) and decreased alertness. In pediatric patients 7 years of age (>20 kg), somnolence appears to occur less frequently (25%). Approximately 16% of patients on brimonidine tartrate ophthalmic solution discontinued from the study due to somnolence. | ||
|useInGeri=No overall differences in safety or effectiveness have been observed between elderly and other adult patients. | |useInGeri=*No overall differences in safety or effectiveness have been observed between elderly and other adult patients. | ||
|useInRenalImpair= | |useInRenalImpair=*Brimonidine has not been studied in patients with [[hepatic impairment]]. | ||
|useInHepaticImpair= | |useInHepaticImpair=*Brimonidine has not been studied in patients with [[renal impairment]]. The effect of [[dialysis]] on brimonidine pharmacokinetics in patients with [[renal failure]] is not known. | ||
|administration=Ophthalmic | |administration=Ophthalmic | ||
|monitoring=FDA | |monitoring=FDA package insert for brimonidine contains no information regarding adverse reactions. | ||
|IVCompat=There is limited information about the IV | |IVCompat=There is limited information about the IV compatibility. | ||
|overdose=Very limited information exists on accidental ingestion of brimonidine in adults; the only adverse reaction reported to date has been hypotension. Symptoms of brimonidine overdose have been reported in neonates, infants, and children receiving | |overdose=*Very limited information exists on accidental ingestion of brimonidine in adults; the only adverse reaction reported to date has been [[hypotension]]. Symptoms of brimonidine overdose have been reported in neonates, infants, and children receiving Brimonidine as part of medical treatment of congenital [[glaucoma]] or by accidental oral ingestion. Treatment of an oral overdose includes supportive and symptomatic therapy; a patent airway should be maintained. | ||
|drugBox={{Drugbox2 | |drugBox={{Drugbox2 | ||
| verifiedrevid = 459982442 | | verifiedrevid = 459982442 | ||
| Line 122: | Line 122: | ||
| StdInChIKey = XYLJNLCSTIOKRM-UHFFFAOYSA-N | | StdInChIKey = XYLJNLCSTIOKRM-UHFFFAOYSA-N | ||
}} | }} | ||
|mechAction= | |mechAction=*Brimonidine is a relatively selective alpha-2 adrenergic receptor agonist with a peak ocular [[hypotensive]] effect occurring at two hours post-dosing. | ||
Fluorophotometric studies in animals and humans suggest that brimonidine tartrate has a dual mechanism of action by reducing aqueous humor production and increasing uveoscleral outflow. | *Fluorophotometric studies in animals and humans suggest that brimonidine tartrate has a dual mechanism of action by reducing [[aqueous humor]] production and increasing uveoscleral outflow. | ||
|structure= | |structure=*Brimonidine (brimonidine tartrate ophthalmic solution) 0.1% or 0.15%, sterile, is a relatively selective alpha-2 adrenergic receptor agonist (topical [[intraocular pressure]] lowering agent). | ||
The structural formula of brimonidine tartrate is: | *The structural formula of brimonidine tartrate is: | ||
[[File:Brimonidine_structure_01.png|thumb|none|400px|This image is provided by the National Library of Medicine.]] | [[File:Brimonidine_structure_01.png|thumb|none|400px|This image is provided by the National Library of Medicine.]] | ||
5-Bromo-6-(2-imidazolidinylideneamino) quinoxaline L-tartrate; MW= 442.24 | *5-Bromo-6-(2-imidazolidinylideneamino) quinoxaline L-tartrate; MW= 442.24 | ||
In solution, | *In solution, Brimonidine (brimonidine tartrate ophthalmic solution) has a clear, greenish-yellow color. It has an osmolality of 250-350 mOsmol/kg and a pH of 7.4-8.0 (0.1%) or 6.9-7.4 (0.15%). | ||
Brimonidine tartrate appears as an off-white to pale-yellow powder and is soluble in both water (0.6 mg/mL) and in the product vehicle (1.4 mg/mL) at pH 7.7. | *Brimonidine tartrate appears as an off-white to pale-yellow powder and is soluble in both water (0.6 mg/mL) and in the product vehicle (1.4 mg/mL) at pH 7.7. | ||
Each mL of | *Each mL of Brimonidine contains the active ingredient brimonidine tartrate 0.1% (1 mg/mL) or 0.15% (1.5 mg/mL) with the inactive ingredients sodium carboxymethylcellulose; sodium borate; boric acid; sodium chloride; potassium chloride; calcium chloride; magnesium chloride; PURITE® 0.005% (0.05 mg/mL) as a preservative; purified water; and hydrochloric acid and/or sodium hydroxide to adjust pH. | ||
|PK='''Absorption''' | |PK='''Absorption''' | ||
After ocular administration of either a 0.1% or 0.2% solution, plasma concentrations peaked within 0.5 to 2.5 hours and declined with a systemic half-life of approximately 2 hours. | *After ocular administration of either a 0.1% or 0.2% solution, plasma concentrations peaked within 0.5 to 2.5 hours and declined with a systemic half-life of approximately 2 hours. | ||
'''Distribution''' | '''Distribution''' | ||
The protein binding of brimonidine has not been studied. | *The protein binding of brimonidine has not been studied. | ||
'''Metabolism''' | '''Metabolism''' | ||
In humans, brimonidine is extensively metabolized by the liver. | *In humans, brimonidine is extensively metabolized by the liver. | ||
'''Excretion''' | '''Excretion''' | ||
Urinary excretion is the major route of elimination of brimonidine and its metabolites. Approximately 87% of an orally-administered radioactive dose of brimonidine was eliminated within 120 hours, with 74% found in the urine. | *Urinary excretion is the major route of elimination of brimonidine and its metabolites. Approximately 87% of an orally-administered radioactive dose of brimonidine was eliminated within 120 hours, with 74% found in the urine. | ||
|nonClinToxic=====Carcinogenesis, Mutagenesis, Impairment of Fertility==== | |nonClinToxic=====Carcinogenesis, Mutagenesis, Impairment of Fertility==== | ||
No compound-related carcinogenic effects were observed in either mice or rats following a 21-month and 24-month study, respectively. In these studies, dietary administration of brimonidine tartrate at doses up to 2.5 mg/kg/day in mice and 1 mg/kg/day in rats achieved 150 and 120 times or 90 and 80 times, respectively, the plasma Cmax drug concentration in humans treated with one drop of | *No compound-related carcinogenic effects were observed in either mice or rats following a 21-month and 24-month study, respectively. In these studies, dietary administration of brimonidine tartrate at doses up to 2.5 mg/kg/day in mice and 1 mg/kg/day in rats achieved 150 and 120 times or 90 and 80 times, respectively, the plasma Cmax drug concentration in humans treated with one drop of Brimonidine 0.1% or 0.15% into both eyes 3 times per day, the recommended daily human dose. | ||
Brimonidine tartrate was not mutagenic or clastogenic in a series of in vitro and in vivo studies including the Ames bacterial reversion test, chromosomal aberration assay in Chinese Hamster Ovary (CHO) cells, and three in vivo studies in CD-1 mice: a host-mediated assay, cytogenetic study, and dominant lethal assay. | *Brimonidine tartrate was not mutagenic or clastogenic in a series of in vitro and in vivo studies including the Ames bacterial reversion test, chromosomal aberration assay in Chinese Hamster Ovary (CHO) cells, and three in vivo studies in CD-1 mice: a host-mediated assay, cytogenetic study, and dominant lethal assay. | ||
Reproduction and fertility studies in rats with brimonidine tartrate demonstrated no adverse effect on male or female fertility at doses which achieve up to approximately 125 and 90 times the systemic exposure following the maximum recommended human ophthalmic dose of | *Reproduction and fertility studies in rats with brimonidine tartrate demonstrated no adverse effect on male or female fertility at doses which achieve up to approximately 125 and 90 times the systemic exposure following the maximum recommended human ophthalmic dose of Brimonidine 0.1% or 0.15%, respectively. | ||
|clinicalStudies=Elevated IOP presents a major risk factor in glaucomatous field loss. The higher the level of IOP, the greater the likelihood of optic nerve damage and visual field loss. Brimonidine tartrate has the action of lowering intraocular pressure with minimal effect on cardiovascular and pulmonary parameters. | |clinicalStudies=*Elevated [[IOP]] presents a major risk factor in glaucomatous field loss. The higher the level of [[IOP]], the greater the likelihood of optic nerve damage and visual field loss. Brimonidine tartrate has the action of lowering [[intraocular pressure]] with minimal effect on cardiovascular and pulmonary parameters. | ||
Clinical studies were conducted to evaluate the safety, efficacy, and acceptability of | *Clinical studies were conducted to evaluate the safety, efficacy, and acceptability of Brimonidine (brimonidine tartrate ophthalmic solution) 0.15% compared with ALPHAGAN® administered three-times-daily in patients with [[open-angle glaucoma]] or [[ocular hypertension]]. Those results indicated that Brimonidine (brimonidine tartrate ophthalmic solution) 0.15% is comparable in [[IOP]] lowering effect to ALPHAGAN® (brimonidine tartrate ophthalmic solution) 0.2%, and effectively lowers [[IOP]] in patients with [[open-angle glaucoma]] or [[ocular hypertension]] by approximately 2-6 mmHg. | ||
A clinical study was conducted to evaluate the safety, efficacy, and acceptability of | *A clinical study was conducted to evaluate the safety, efficacy, and acceptability of Brimonidine (brimonidine tartrate ophthalmic solution) 0.1% compared with ALPHAGAN® administered three-times-daily in patients with [[open-angle glaucoma]] or ocular [[hypertension]]. Those results indicated that Brimonidine (brimonidine tartrate ophthalmic solution) 0.1% is equivalent in [[IOP]] lowering effect to ALPHAGAN® (brimonidine tartrate ophthalmic solution) 0.2%, and effectively lowers [[IOP]] in patients with [[open-angle glaucoma]] or [[ocular hypertension]] by approximately 2-6 mmHg. | ||
|howSupplied= | |howSupplied=*Brimonidine is supplied sterile, in teal opaque plastic LDPE bottles and tips, with purple high impact polystyrene (HIPS) caps as follows: | ||
0.1% | *0.1% | ||
5 mL in 10 mL bottle NDC 0023-9321-05 | :*5 mL in 10 mL bottle NDC 0023-9321-05 | ||
10 mL in 10 mL bottle NDC 0023-9321-10 | :*10 mL in 10 mL bottle NDC 0023-9321-10 | ||
15 mL in 15 mL bottle NDC 0023-9321-15 | :*15 mL in 15 mL bottle NDC 0023-9321-15 | ||
0.15% | *0.15% | ||
5 mL in 10 mL bottle NDC 0023-9177-05 | :*5 mL in 10 mL bottle NDC 0023-9177-05 | ||
10 mL in 10 mL bottle NDC 0023-9177-10 | :*10 mL in 10 mL bottle NDC 0023-9177-10 | ||
15 mL in 15 mL bottle NDC 0023-9177-15 | :*15 mL in 15 mL bottle NDC 0023-9177-15 | ||
|storage=Storage: Store at 15°-25°C (59°-77°F). | |storage=Storage: Store at 15°-25°C (59°-77°F). | ||
|fdaPatientInfo=Patients should be instructed that ocular solutions, if handled improperly or if the tip of the dispensing container contacts the eye or surrounding structures, can become contaminated by common bacteria known to cause ocular infections. Serious damage to the eye and subsequent loss of vision may result from using contaminated solutions | |fdaPatientInfo=*Patients should be instructed that ocular solutions, if handled improperly or if the tip of the dispensing container contacts the eye or surrounding structures, can become contaminated by common bacteria known to cause ocular infections. Serious damage to the eye and subsequent loss of vision may result from using contaminated solutions. Always replace the cap after using. If solution changes color or becomes cloudy, do not use. Do not use the product after the expiration date marked on the bottle. | ||
Patients also should be advised that if they have ocular surgery or develop an intercurrent ocular condition (e.g., trauma or infection), they should immediately seek their physician's advice concerning the continued use of the present multidose container. | *Patients also should be advised that if they have ocular surgery or develop an intercurrent ocular condition (e.g., trauma or infection), they should immediately seek their physician's advice concerning the continued use of the present multidose container. | ||
If more than one topical ophthalmic drug is being used, the drugs should be administered at least five minutes apart. | *If more than one topical ophthalmic drug is being used, the drugs should be administered at least five minutes apart. | ||
As with other similar medications, | *As with other similar medications, Brimonidine may cause fatigue and/or drowsiness in some patients. Patients who engage in hazardous activities should be cautioned of the potential for a decrease in mental alertness. | ||
|alcohol=Alcohol-Brimonidine interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication. | |alcohol=Alcohol-Brimonidine interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication. | ||
|brandNames=* ALPHAGAN P | |brandNames=* ALPHAGAN P | ||
Latest revision as of 06:40, 18 May 2015
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Sheng Shi, M.D. [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Brimonidine (ophthalmic) is an alpha-2 adrenergic agonist that is FDA approved for the treatment of open-angle glaucoma or ocular hypertension. Common adverse reactions include hypertension, contact dermatitis, erythema, flushing, sensation of burning of skin, xerostomia, somnolence, allergic conjunctivitis, burning sensation in eye, conjunctival discoloration, conjunctival hyperemia, follicular conjunctivitis, acute hypersensitivity reaction, itching of eye, lid retraction, and visual disturbance.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Ocular Hypertension
- Dosing information
- Recommended dose is one drop three times daily, approximately 8 hours apart. Brimonidine ophthalmic solution may be used concomitantly with other topical ophthalmic drug products to lower intraocular pressure. If more than one topical ophthalmic product is to be used, the different products should be instilled at least 5 minutes apart.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of brimonidine in adult patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of brimonidine in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
There is limited information regarding Brimonidine (ophthalmic) FDA-Labeled Indications and Dosage (Pediatric) in the drug label.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of brimonidine in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of brimonidine in pediatric patients.
Contraindications
Neonates and Infants (under the age of 2 years)
- Brimonidine is contraindicated in neonates and infants (under the age of 2 years).
Hypersensitivity Reactions
- Brimonidine is contraindicated in patients who have exhibited a hypersensitivity reaction to any component of this medication in the past.
Warnings
Potentiation of Vascular Insufficiency
- Brimonidine may potentiate syndromes associated with vascular insufficiency. Brimonidine should be used with caution in patients with depression, cerebral or coronary insufficiency, Raynaud's phenomenon, orthostatic hypotension, or thromboangiitis obliterans.
Severe Cardiovascular Disease
- Although brimonidine tartrate ophthalmic solution had minimal effect on the blood pressure of patients in clinical studies, caution should be exercised in treating patients with severe cardiovascular disease.
Contamination of Topical Ophthalmic Products After Use
- There have been reports of bacterial keratitis associated with the use of multiple-dose containers of topical ophthalmic products. These containers had been inadvertently contaminated by patients who, in most cases, had a concurrent corneal disease or a disruption of the ocular epithelial surface.
Adverse Reactions
Clinical Trials Experience
- Because clinical studies are conducted under widely varying conditions, adverse reaction rates observed in the clinical studies of a drug cannot be directly compared to rates in the clinical studies of another drug and may not reflect the rates observed in practice.
- Adverse reactions occurring in approximately 10-20% of the subjects receiving brimonidine ophthalmic solution (0.1-0.2%) included: allergic conjunctivitis, conjunctival hyperemia, and eye pruritus. Adverse reactions occurring in approximately 5-9% included: burning sensation, conjunctival folliculosis, hypertension, ocular allergic reaction, oral dryness, and visual disturbance.
- Adverse reactions occurring in approximately 1-4% of the subjects receiving brimonidine ophthalmic solution (0.1-0.2%) included: abnormal taste, allergic reaction, asthenia, blepharitis, blepharoconjunctivitis, blurred vision, bronchitis, cataract, conjunctival edema, conjunctival hemorrhage, conjunctivitis, cough, dizziness, dyspepsia, dyspnea, epiphora, eye discharge, eye dryness, eye irritation, eye pain, eyelid edema, eyelid erythema, fatigue, flu syndrome, follicular conjunctivitis, foreign body sensation, gastrointestinal disorder, headache, hypercholesterolemia, hypotension, infection (primarily colds and respiratory infections), insomnia, keratitis, lid disorder, pharyngitis, photophobia, rash, rhinitis, sinus infection, sinusitis, somnolence, stinging, superficial punctate keratopathy, tearing, visual field defect, vitreous detachment, vitreous disorder, vitreous floaters, and worsened visual acuity.
- The following reactions were reported in less than 1% of subjects: corneal erosion, hordeolum, nasal dryness, and taste perversion.
Postmarketing Experience
- The following reactions have been identified during postmarketing use of brimonidine tartrate ophthalmic solutions in clinical practice. Because they are reported voluntarily from a population of unknown size, estimates of frequency cannot be made. The reactions, which have been chosen for inclusion due to either their seriousness, frequency of reporting, possible causal connection to brimonidine tartrate ophthalmic solutions, or a combination of these factors, include: bradycardia, depression, hypersensitivity, iritis, keratoconjunctivitis sicca, miosis, nausea, skin reactions (including erythema, eyelid pruritus, rash, and vasodilation), syncope, and tachycardia. Apnea, bradycardia, coma, hypotension, hypothermia, hypotonia, lethargy, pallor, respiratory depression, and somnolence have been reported in infants receiving brimonidine tartrate ophthalmic solutions.
Drug Interactions
Antihypertensives/Cardiac Glycosides
- Because Brimonidine may reduce blood pressure, caution in using drugs such as antihypertensives and/or cardiac glycosides with Brimonidine is advised.
CNS Depressants
- Although specific drug interaction studies have not been conducted with Brimonidine, the possibility of an additive or potentiating effect with CNS depressants (alcohol, barbiturates, opiates, sedatives, or anesthetics) should be considered.
Tricyclic Antidepressants
- Tricyclic antidepressants have been reported to blunt the hypotensive effect of systemic clonidine. It is not known whether the concurrent use of these agents with Brimonidine in humans can lead to resulting interference with the IOP lowering effect. Caution is advised in patients taking tricyclic antidepressants which can affect the metabolism and uptake of circulating amines.
Monoamine Oxidase Inhibitors
- Monoamine oxidase (MAO) inhibitors may theoretically interfere with the metabolism of brimonidine and potentially result in an increased systemic side-effect such as hypotension. Caution is advised in patients taking MAO inhibitors which can affect the metabolism and uptake of circulating amines.
Use in Specific Populations
Pregnancy
- Teratogenicity studies have been performed in animals.
- Brimonidine tartrate was not teratogenic when given orally during gestation days 6 through 15 in rats and days 6 through 18 in rabbits. The highest doses of brimonidine tartrate in rats (2.5 mg/kg/day) and rabbits (5.0 mg/kg/day) achieved AUC exposure values 360- and 20-fold higher, or 260- and 15-fold higher, respectively, than similar values estimated in humans treated with Brimonidine 0.1% or 0.15%, 1 drop in both eyes three times daily.
- There are no adequate and well-controlled studies in pregnant women; however, in animal studies, brimonidine crossed the placenta and entered into the fetal circulation to a limited extent. Because animal reproduction studies are not always predictive of human response, Brimonidine should be used during pregnancy only if the potential benefit to the mother justifies the potential risk to the fetus.
Pregnancy Category (AUS):
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Brimonidine (ophthalmic) in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Brimonidine (ophthalmic) during labor and delivery.
Nursing Mothers
- It is not known whether brimonidine tartrate is excreted in human milk, although in animal studies, brimonidine tartrate has been shown to be excreted in breast milk. Because of the potential for serious adverse reactions from Brimonidine in nursing infants, a decision should be made whether to discontinue nursing or to discontinue the drug, taking into account the importance of the drug to the mother.
Pediatric Use
- Brimonidine is contraindicated in children under the age of 2 years. During postmarketing surveillance, apnea, bradycardia, coma, hypotension, hypothermia, hypotonia, lethargy, pallor, respiratory depression, and somnolence have been reported in infants receiving brimonidine. The safety and effectiveness of brimonidine tartrate have not been studied in children below the age of 2 years.
- In a well-controlled clinical study conducted in pediatric glaucoma patients (ages 2 to 7 years) the most commonly observed adverse reactions with brimonidine tartrate ophthalmic solution 0.2% dosed three times daily were somnolence (50-83% in patients ages 2 to 6 years) and decreased alertness. In pediatric patients 7 years of age (>20 kg), somnolence appears to occur less frequently (25%). Approximately 16% of patients on brimonidine tartrate ophthalmic solution discontinued from the study due to somnolence.
Geriatic Use
- No overall differences in safety or effectiveness have been observed between elderly and other adult patients.
Gender
There is no FDA guidance on the use of Brimonidine (ophthalmic) with respect to specific gender populations.
Race
There is no FDA guidance on the use of Brimonidine (ophthalmic) with respect to specific racial populations.
Renal Impairment
- Brimonidine has not been studied in patients with hepatic impairment.
Hepatic Impairment
- Brimonidine has not been studied in patients with renal impairment. The effect of dialysis on brimonidine pharmacokinetics in patients with renal failure is not known.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Brimonidine (ophthalmic) in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Brimonidine (ophthalmic) in patients who are immunocompromised.
Administration and Monitoring
Administration
Ophthalmic
Monitoring
FDA package insert for brimonidine contains no information regarding adverse reactions.
IV Compatibility
There is limited information about the IV compatibility.
Overdosage
- Very limited information exists on accidental ingestion of brimonidine in adults; the only adverse reaction reported to date has been hypotension. Symptoms of brimonidine overdose have been reported in neonates, infants, and children receiving Brimonidine as part of medical treatment of congenital glaucoma or by accidental oral ingestion. Treatment of an oral overdose includes supportive and symptomatic therapy; a patent airway should be maintained.
Pharmacology
| |
Brimonidine (ophthalmic)
| |
| Systematic (IUPAC) name | |
| 5-Bromo-N-(4,5-dihydro-1H-imidazol-2-yl) quinoxalin-6-amine | |
| Identifiers | |
| CAS number | |
| ATC code | D11 S01EA05 (WHO) |
| PubChem | |
| DrugBank | |
| Chemical data | |
| Formula | Template:OrganicBox atomTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox |
| Mol. mass | 292.135 g/mol |
| SMILES | & |
| Pharmacokinetic data | |
| Bioavailability | ? |
| Metabolism | Primarily liver |
| Half life | 3 hours ocular 12 hours topical |
| Excretion | ? |
| Therapeutic considerations | |
| Pregnancy cat. |
B(US) |
| Legal status |
[[Prescription drug|Template:Unicode-only]](US) |
| Routes | Ocular (eye drops), topical (gel) |
Mechanism of Action
- Brimonidine is a relatively selective alpha-2 adrenergic receptor agonist with a peak ocular hypotensive effect occurring at two hours post-dosing.
- Fluorophotometric studies in animals and humans suggest that brimonidine tartrate has a dual mechanism of action by reducing aqueous humor production and increasing uveoscleral outflow.
Structure
- Brimonidine (brimonidine tartrate ophthalmic solution) 0.1% or 0.15%, sterile, is a relatively selective alpha-2 adrenergic receptor agonist (topical intraocular pressure lowering agent).
- The structural formula of brimonidine tartrate is:
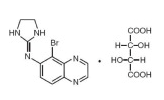
- 5-Bromo-6-(2-imidazolidinylideneamino) quinoxaline L-tartrate; MW= 442.24
- In solution, Brimonidine (brimonidine tartrate ophthalmic solution) has a clear, greenish-yellow color. It has an osmolality of 250-350 mOsmol/kg and a pH of 7.4-8.0 (0.1%) or 6.9-7.4 (0.15%).
- Brimonidine tartrate appears as an off-white to pale-yellow powder and is soluble in both water (0.6 mg/mL) and in the product vehicle (1.4 mg/mL) at pH 7.7.
- Each mL of Brimonidine contains the active ingredient brimonidine tartrate 0.1% (1 mg/mL) or 0.15% (1.5 mg/mL) with the inactive ingredients sodium carboxymethylcellulose; sodium borate; boric acid; sodium chloride; potassium chloride; calcium chloride; magnesium chloride; PURITE® 0.005% (0.05 mg/mL) as a preservative; purified water; and hydrochloric acid and/or sodium hydroxide to adjust pH.
Pharmacodynamics
There is limited information regarding Brimonidine (ophthalmic) Pharmacodynamics in the drug label.
Pharmacokinetics
Absorption
- After ocular administration of either a 0.1% or 0.2% solution, plasma concentrations peaked within 0.5 to 2.5 hours and declined with a systemic half-life of approximately 2 hours.
Distribution
- The protein binding of brimonidine has not been studied.
Metabolism
- In humans, brimonidine is extensively metabolized by the liver.
Excretion
- Urinary excretion is the major route of elimination of brimonidine and its metabolites. Approximately 87% of an orally-administered radioactive dose of brimonidine was eliminated within 120 hours, with 74% found in the urine.
Nonclinical Toxicology
Carcinogenesis, Mutagenesis, Impairment of Fertility
- No compound-related carcinogenic effects were observed in either mice or rats following a 21-month and 24-month study, respectively. In these studies, dietary administration of brimonidine tartrate at doses up to 2.5 mg/kg/day in mice and 1 mg/kg/day in rats achieved 150 and 120 times or 90 and 80 times, respectively, the plasma Cmax drug concentration in humans treated with one drop of Brimonidine 0.1% or 0.15% into both eyes 3 times per day, the recommended daily human dose.
- Brimonidine tartrate was not mutagenic or clastogenic in a series of in vitro and in vivo studies including the Ames bacterial reversion test, chromosomal aberration assay in Chinese Hamster Ovary (CHO) cells, and three in vivo studies in CD-1 mice: a host-mediated assay, cytogenetic study, and dominant lethal assay.
- Reproduction and fertility studies in rats with brimonidine tartrate demonstrated no adverse effect on male or female fertility at doses which achieve up to approximately 125 and 90 times the systemic exposure following the maximum recommended human ophthalmic dose of Brimonidine 0.1% or 0.15%, respectively.
Clinical Studies
- Elevated IOP presents a major risk factor in glaucomatous field loss. The higher the level of IOP, the greater the likelihood of optic nerve damage and visual field loss. Brimonidine tartrate has the action of lowering intraocular pressure with minimal effect on cardiovascular and pulmonary parameters.
- Clinical studies were conducted to evaluate the safety, efficacy, and acceptability of Brimonidine (brimonidine tartrate ophthalmic solution) 0.15% compared with ALPHAGAN® administered three-times-daily in patients with open-angle glaucoma or ocular hypertension. Those results indicated that Brimonidine (brimonidine tartrate ophthalmic solution) 0.15% is comparable in IOP lowering effect to ALPHAGAN® (brimonidine tartrate ophthalmic solution) 0.2%, and effectively lowers IOP in patients with open-angle glaucoma or ocular hypertension by approximately 2-6 mmHg.
- A clinical study was conducted to evaluate the safety, efficacy, and acceptability of Brimonidine (brimonidine tartrate ophthalmic solution) 0.1% compared with ALPHAGAN® administered three-times-daily in patients with open-angle glaucoma or ocular hypertension. Those results indicated that Brimonidine (brimonidine tartrate ophthalmic solution) 0.1% is equivalent in IOP lowering effect to ALPHAGAN® (brimonidine tartrate ophthalmic solution) 0.2%, and effectively lowers IOP in patients with open-angle glaucoma or ocular hypertension by approximately 2-6 mmHg.
How Supplied
- Brimonidine is supplied sterile, in teal opaque plastic LDPE bottles and tips, with purple high impact polystyrene (HIPS) caps as follows:
- 0.1%
- 5 mL in 10 mL bottle NDC 0023-9321-05
- 10 mL in 10 mL bottle NDC 0023-9321-10
- 15 mL in 15 mL bottle NDC 0023-9321-15
- 0.15%
- 5 mL in 10 mL bottle NDC 0023-9177-05
- 10 mL in 10 mL bottle NDC 0023-9177-10
- 15 mL in 15 mL bottle NDC 0023-9177-15
Storage
Storage: Store at 15°-25°C (59°-77°F).
Images
Drug Images
{{#ask: Page Name::Brimonidine (ophthalmic) |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
{{#ask: Label Page::Brimonidine (ophthalmic) |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
- Patients should be instructed that ocular solutions, if handled improperly or if the tip of the dispensing container contacts the eye or surrounding structures, can become contaminated by common bacteria known to cause ocular infections. Serious damage to the eye and subsequent loss of vision may result from using contaminated solutions. Always replace the cap after using. If solution changes color or becomes cloudy, do not use. Do not use the product after the expiration date marked on the bottle.
- Patients also should be advised that if they have ocular surgery or develop an intercurrent ocular condition (e.g., trauma or infection), they should immediately seek their physician's advice concerning the continued use of the present multidose container.
- If more than one topical ophthalmic drug is being used, the drugs should be administered at least five minutes apart.
- As with other similar medications, Brimonidine may cause fatigue and/or drowsiness in some patients. Patients who engage in hazardous activities should be cautioned of the potential for a decrease in mental alertness.
Precautions with Alcohol
Alcohol-Brimonidine interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
- ALPHAGAN P
- Mirvaso
Look-Alike Drug Names
There is limited information about the look-alike drug names.
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
{{#subobject:
|Label Page=Brimonidine (ophthalmic) |Label Name=Brimonidine_label_01.jpg
}}
{{#subobject:
|Label Page=Brimonidine (ophthalmic) |Label Name=Brimonidine_label_02.jpg
}}
{{#subobject:
|Label Page=Brimonidine (ophthalmic) |Label Name=Brimonidine_panel_01.png
}}
{{#subobject:
|Label Page=Brimonidine (ophthalmic) |Label Name=Brimonidine_panel_02.png
}}