Acute respiratory distress syndrome pathophysiology: Difference between revisions
(Created page with "{{Acute respiratory distress syndrome}} {{CMG}} ==Overview== Acute respiratory distress syndrome primarily results from the diffuse inflammation of lung parenchyma. Loss of aera...") |
Gerald Chi- (talk | contribs) |
||
| (14 intermediate revisions by 5 users not shown) | |||
| Line 1: | Line 1: | ||
__NOTOC__ | |||
{{Acute respiratory distress syndrome}} | {{Acute respiratory distress syndrome}} | ||
{{CMG}} | {{CMG}}; {{AE}} {{BShaller}} | ||
==Overview== | ==Overview== | ||
ARDS is a syndrome of [[inflammation]] and increased [[permeability]] with the lung parenchyma that leads to loss of [[pneumocyte|type I pneumocytes]], impaired [[gas exchange]], inappropriate [[cell proliferation]] within [[alveolus|alveoli]], and, in survivors, [[fibrosis]]. | |||
==Pathophysiology== | ==Pathophysiology== | ||
ARDS typically develops within 24 to 48 hours of the provoking illness or injury and is classically divided into three phases:<ref>Katzenstein, A. L., C. M. Bloor, and A. A. Leibow. “Diffuse Alveolar Damage--the Role of Oxygen, Shock, and Related Factors. A Review.” The American Journal of Pathology 85, no. 1 (October 1976): 209–28.</ref><ref>Tomashefski, J. F. “Pulmonary Pathology of Acute Respiratory Distress Syndrome.” Clinics in Chest Medicine 21, no. 3 (September 2000): 435–66.</ref><ref>Thille, Arnaud W., Andrés Esteban, Pilar Fernández-Segoviano, José-María Rodriguez, José-Antonio Aramburu, Patricio Vargas-Errázuriz, Ana Martín-Pellicer, José A. Lorente, and Fernando Frutos-Vivar. “Chronology of Histological Lesions in Acute Respiratory Distress Syndrome with Diffuse Alveolar Damage: A Prospective Cohort Study of Clinical Autopsies.” The Lancet. Respiratory Medicine 1, no. 5 (July 2013): 395–401. doi:10.1016/S2213-2600(13)70053-5.</ref> | |||
*'''Exudative phase (''within 5 to 7 days'')''': Systemic inflammation results in increased permeability of the [[alveolar-capillary barrier]] and leads to the formation of hyaline membranes along alveolar walls, accumulation of proteinaceous [[exudate]] within the [[Pulmonary alveolus|alveolar air spaces]] (''non-cardiogenic [[pulmonary edema]]''), and extravasation of inflammatory cells (predominantly [[neutrophils|neutrophils]]) into the lung parenchyma, leading to extensive alveolar damage and, occasionally, diffuse alveolar hemorrhage | |||
* | *'''Proliferative phase (''within 7 to 21 days'')''': [[Fibroblast]] proliferation, [[collagen]] deposition, and early [[fibrosis|fibrotic changes]] are observed within the pulmonary [[interstitium]] as alveolar exudate and hyaline membranes begin to be absorbed | ||
* [[ | *'''Fibrotic phase (''within several weeks'')''': Many patients with ARDS will develop some degree of pulmonary [[fibrosis]], of which at least one-quarter will go on to develop clinically apparent [[interstitial lung disease|fibrotic lung disease]] with a [[spirometry#restrictive lung patterns|restrictive ventilatory defect]] on [[pulmonary function tests]];<ref name="pmid23520315">{{cite journal| author=Burnham EL, Janssen WJ, Riches DW, Moss M, Downey GP| title=The fibroproliferative response in acute respiratory distress syndrome: mechanisms and clinical significance. | journal=Eur Respir J | year= 2014 | volume= 43 | issue= 1 | pages= 276-85 | pmid=23520315 | doi=10.1183/09031936.00196412 | pmc=4015132 | url=http://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=23520315 }} </ref> the development and extent of pulmonary fibrosis in ARDS correlates with an increased mortality risk<ref name="pmid7813276">{{cite journal| author=Martin C, Papazian L, Payan MJ, Saux P, Gouin F| title=Pulmonary fibrosis correlates with outcome in adult respiratory distress syndrome. A study in mechanically ventilated patients. | journal=Chest | year= 1995 | volume= 107 | issue= 1 | pages= 196-200 | pmid=7813276 | doi= | pmc= | url=http://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=7813276 }} </ref> | ||
* | |||
=== | ===Genetics=== | ||
The role of [[genetics]] in the development of ARDS is an ongoing area of research. While studies have demonstrated associations between certain genetic factors (including [[single nucleotide polymorphism]]s and [[allele|allelic variants]] of [[angiotensin-converting enzyme|angiotensin-converting enzyme]]) and increased susceptibility to the development of ARDS, the nature and implications of these relationships remain uncertain.<ref name="pmid16484896">{{cite journal| author=Jerng JS, Yu CJ, Wang HC, Chen KY, Cheng SL, Yang PC| title=Polymorphism of the angiotensin-converting enzyme gene affects the outcome of acute respiratory distress syndrome. | journal=Crit Care Med | year= 2006 | volume= 34 | issue= 4 | pages= 1001-6 | pmid=16484896 | doi=10.1097/01.CCM.0000206107.92476.39 | pmc= | url=http://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=16484896 }} </ref><ref name="pmid23364437">{{cite journal| author=Cardinal-Fernández P, Ferruelo A, El-Assar M, Santiago C, Gómez-Gallego F, Martín-Pellicer A et al.| title=Genetic predisposition to acute respiratory distress syndrome in patients with severe sepsis. | journal=Shock | year= 2013 | volume= 39 | issue= 3 | pages= 255-60 | pmid=23364437 | doi=10.1097/SHK.0b013e3182866ff9 | pmc= | url=http://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=23364437 }} </ref><ref name="pmid23048207">{{cite journal| author=Tejera P, Meyer NJ, Chen F, Feng R, Zhao Y, O'Mahony DS et al.| title=Distinct and replicable genetic risk factors for acute respiratory distress syndrome of pulmonary or extrapulmonary origin. | journal=J Med Genet | year= 2012 | volume= 49 | issue= 11 | pages= 671-80 | pmid=23048207 | doi=10.1136/jmedgenet-2012-100972 | pmc=3654537 | url=http://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=23048207 }} </ref> | |||
===Pathology=== | |||
On gross pathology, the following are characteristic findings of ARDS: | |||
*Firm, boggy, and dusky lungs | |||
*Generally increased weight compared to healthy lungs due to [[edema]] | |||
* | |||
* | On microscopic histopathological analysis, the following are characteristic findings of ARDS: | ||
* | *Lung parenchyma demonstrates: | ||
* | **Hyaline membranes lining the alveolar air spaces | ||
* | **Edema fluid within alveoli and the interstitium | ||
* | **Shedding of [[pneumocyte|type I pneumocytes]] and proliferation of [[pneumocyte|type II pneumocytes]] | ||
* | **Infiltration of [[neutrophil|polymorphonuclear]] and other [[white blood cells|inflammatory cells]] into the interstitial and alveolar compartments, | ||
**[[Thrombosis]] and obliteration of pulmonary [[capillaries]] | |||
**[[Hemorrhage]] into alveoli | |||
*Features specific to the underlying disease process (e.g., [[pneumonia]] or [[aspiration pneumonia|aspiration pneumonitis]]) | |||
*With progression, alveolar infiltrates are reabsorbed and the inflammatory milieu is replaced by increased [[collagen]] deposition and proliferating [[fibroblast]]s, culminating in [[interstitial fibrosis]] | |||
[[File:Hyaline membranes - very high mag.jpg|thumb|left|Hyaline membranes - very high magnification]] | |||
<br><br><br><br><br><br><br><br><br><br><br> | |||
==References== | ==References== | ||
{{ | {{reflist|2}} | ||
[[Category:Pulmonology]] | [[Category:Pulmonology]] | ||
Latest revision as of 03:53, 20 July 2016
|
Acute respiratory distress syndrome Microchapters |
|
Differentiating Acute respiratory distress syndrome from other Diseases |
|---|
|
Diagnosis |
|
Treatment |
|
Case Studies |
|
Acute respiratory distress syndrome pathophysiology On the Web |
|
American Roentgen Ray Society Images of Acute respiratory distress syndrome pathophysiology |
|
Acute respiratory distress syndrome pathophysiology in the news |
|
Blogs on Acute respiratory distress syndrome pathophysiology |
|
Directions to Hospitals Treating Acute respiratory distress syndrome |
|
Risk calculators and risk factors for Acute respiratory distress syndrome pathophysiology |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Brian Shaller, M.D. [2]
Overview
ARDS is a syndrome of inflammation and increased permeability with the lung parenchyma that leads to loss of type I pneumocytes, impaired gas exchange, inappropriate cell proliferation within alveoli, and, in survivors, fibrosis.
Pathophysiology
ARDS typically develops within 24 to 48 hours of the provoking illness or injury and is classically divided into three phases:[1][2][3]
- Exudative phase (within 5 to 7 days): Systemic inflammation results in increased permeability of the alveolar-capillary barrier and leads to the formation of hyaline membranes along alveolar walls, accumulation of proteinaceous exudate within the alveolar air spaces (non-cardiogenic pulmonary edema), and extravasation of inflammatory cells (predominantly neutrophils) into the lung parenchyma, leading to extensive alveolar damage and, occasionally, diffuse alveolar hemorrhage
- Proliferative phase (within 7 to 21 days): Fibroblast proliferation, collagen deposition, and early fibrotic changes are observed within the pulmonary interstitium as alveolar exudate and hyaline membranes begin to be absorbed
- Fibrotic phase (within several weeks): Many patients with ARDS will develop some degree of pulmonary fibrosis, of which at least one-quarter will go on to develop clinically apparent fibrotic lung disease with a restrictive ventilatory defect on pulmonary function tests;[4] the development and extent of pulmonary fibrosis in ARDS correlates with an increased mortality risk[5]
Genetics
The role of genetics in the development of ARDS is an ongoing area of research. While studies have demonstrated associations between certain genetic factors (including single nucleotide polymorphisms and allelic variants of angiotensin-converting enzyme) and increased susceptibility to the development of ARDS, the nature and implications of these relationships remain uncertain.[6][7][8]
Pathology
On gross pathology, the following are characteristic findings of ARDS:
- Firm, boggy, and dusky lungs
- Generally increased weight compared to healthy lungs due to edema
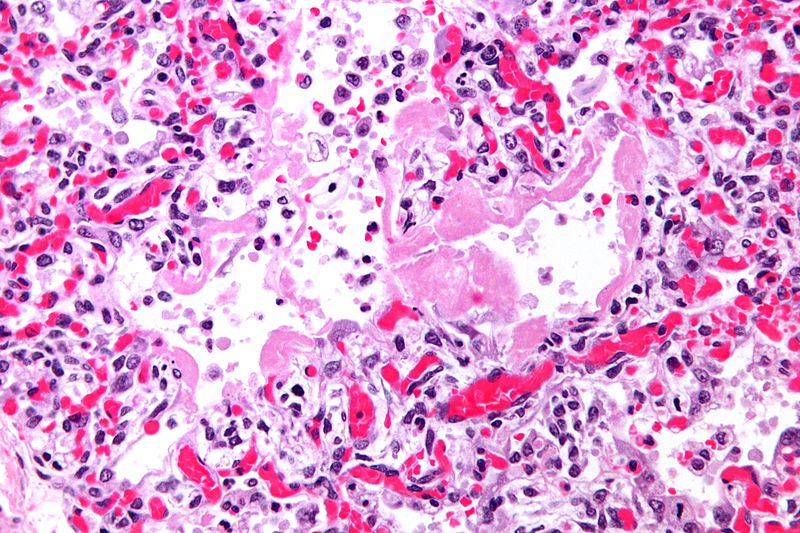
On microscopic histopathological analysis, the following are characteristic findings of ARDS:
- Lung parenchyma demonstrates:
- Hyaline membranes lining the alveolar air spaces
- Edema fluid within alveoli and the interstitium
- Shedding of type I pneumocytes and proliferation of type II pneumocytes
- Infiltration of polymorphonuclear and other inflammatory cells into the interstitial and alveolar compartments,
- Thrombosis and obliteration of pulmonary capillaries
- Hemorrhage into alveoli
- Features specific to the underlying disease process (e.g., pneumonia or aspiration pneumonitis)
- With progression, alveolar infiltrates are reabsorbed and the inflammatory milieu is replaced by increased collagen deposition and proliferating fibroblasts, culminating in interstitial fibrosis
References
- ↑ Katzenstein, A. L., C. M. Bloor, and A. A. Leibow. “Diffuse Alveolar Damage--the Role of Oxygen, Shock, and Related Factors. A Review.” The American Journal of Pathology 85, no. 1 (October 1976): 209–28.
- ↑ Tomashefski, J. F. “Pulmonary Pathology of Acute Respiratory Distress Syndrome.” Clinics in Chest Medicine 21, no. 3 (September 2000): 435–66.
- ↑ Thille, Arnaud W., Andrés Esteban, Pilar Fernández-Segoviano, José-María Rodriguez, José-Antonio Aramburu, Patricio Vargas-Errázuriz, Ana Martín-Pellicer, José A. Lorente, and Fernando Frutos-Vivar. “Chronology of Histological Lesions in Acute Respiratory Distress Syndrome with Diffuse Alveolar Damage: A Prospective Cohort Study of Clinical Autopsies.” The Lancet. Respiratory Medicine 1, no. 5 (July 2013): 395–401. doi:10.1016/S2213-2600(13)70053-5.
- ↑ Burnham EL, Janssen WJ, Riches DW, Moss M, Downey GP (2014). "The fibroproliferative response in acute respiratory distress syndrome: mechanisms and clinical significance". Eur Respir J. 43 (1): 276–85. doi:10.1183/09031936.00196412. PMC 4015132. PMID 23520315.
- ↑ Martin C, Papazian L, Payan MJ, Saux P, Gouin F (1995). "Pulmonary fibrosis correlates with outcome in adult respiratory distress syndrome. A study in mechanically ventilated patients". Chest. 107 (1): 196–200. PMID 7813276.
- ↑ Jerng JS, Yu CJ, Wang HC, Chen KY, Cheng SL, Yang PC (2006). "Polymorphism of the angiotensin-converting enzyme gene affects the outcome of acute respiratory distress syndrome". Crit Care Med. 34 (4): 1001–6. doi:10.1097/01.CCM.0000206107.92476.39. PMID 16484896.
- ↑ Cardinal-Fernández P, Ferruelo A, El-Assar M, Santiago C, Gómez-Gallego F, Martín-Pellicer A; et al. (2013). "Genetic predisposition to acute respiratory distress syndrome in patients with severe sepsis". Shock. 39 (3): 255–60. doi:10.1097/SHK.0b013e3182866ff9. PMID 23364437.
- ↑ Tejera P, Meyer NJ, Chen F, Feng R, Zhao Y, O'Mahony DS; et al. (2012). "Distinct and replicable genetic risk factors for acute respiratory distress syndrome of pulmonary or extrapulmonary origin". J Med Genet. 49 (11): 671–80. doi:10.1136/jmedgenet-2012-100972. PMC 3654537. PMID 23048207.