Voriconazole (injection)
{{DrugProjectFormSinglePage |authorTag=Alberto Plate [1] |genericName=Voriconazole |aOrAn=an |drugClass=antifungal triazole |indicationType=treatment |indication=invasive aspergillosis, candidemia in non-neutropenic patients, esophageal candidiasis, serious fungal infections caused by Scedosporium apiospermum and Fusarium spp. including fusarium solani, in patients intolerant of, or refractory to, other therapy |blackBoxWarningTitle=TITLE |blackBoxWarningBody=Condition Name: (Content) |offLabelAdultGuideSupport=There is limited information regarding Off-Label Guideline-Supported Use of Voriconazole in adult patients. |offLabelAdultNoGuideSupport=There is limited information regarding Off-Label Non–Guideline-Supported Use of Voriconazole in adult patients. |offLabelPedGuideSupport=There is limited information regarding Off-Label Guideline-Supported Use of Voriconazole in pediatric patients. |offLabelPedNoGuideSupport=There is limited information regarding Off-Label Non–Guideline-Supported Use of Voriconazole in pediatric patients. |contraindications=*VFEND is contraindicated in patients with known hypersensitivity to voriconazole or its excipients. There is no information regarding cross-sensitivity between VFEND (voriconazole) and other azole antifungal agents. Caution should be used when prescribing VFEND to patients with hypersensitivity to other azoles.
- Coadministration of terfenadine, astemizole, cisapride, pimozide or quinidine with VFEND is contraindicated because increased plasma concentrations of these drugs can lead to QT prolongation and rare occurrences of torsade de pointes.
- Coadministration of VFEND with sirolimus is contraindicated because VFEND significantly increases sirolimus concentrations
- Coadministration of VFEND with rifampin, carbamazepine and long-acting barbiturates is contraindicated because these drugs are likely to decrease plasma voriconazole concentrations significantly.
- Coadministration of standard doses of voriconazole with efavirenz doses of 400 mg q24h or higher is contraindicated, because efavirenz significantly decreases plasma voriconazole concentrations in healthy subjects at these doses. *Voriconazole also significantly increases efavirenz plasma concentrations.
- Coadministration of VFEND with high-dose ritonavir (400 mg q12h) is contraindicated because ritonavir (400 mg q12h) significantly decreases plasma voriconazole concentrations. Coadministration of voriconazole and low-dose ritonavir (100 mg q12h) should be avoided, unless an assessment of the benefit/risk to the patient justifies the use of voriconazole.
- Coadministration of VFEND with rifabutin is contraindicated since VFEND significantly increases rifabutin plasma concentrations and rifabutin also significantly decreases voriconazole plasma concentrations.
- Coadministration of VFEND with ergot alkaloids (ergotamine and dihydroergotamine) is contraindicated because VFEND may increase the plasma concentration of ergot alkaloids, which may lead to ergotism.
- Coadministration of VFEND with St. John's Wort is contraindicated because this herbal supplement may decrease voriconazole plasma concentration.
|warnings======Hepatic Toxicity===== In clinical trials, there have been uncommon cases of serious hepatic reactions during treatment with VFEND (including clinical hepatitis, cholestasis and fulminant hepatic failure, including fatalities). Instances of hepatic reactions were noted to occur primarily in patients with serious underlying medical conditions (predominantly hematological malignancy). Hepatic reactions, including hepatitis and jaundice, have occurred among patients with no other identifiable risk factors. Liver dysfunction has usually been reversible on discontinuation of therapy.
- Monitoring of hepatic function Liver function tests should be evaluated at the start of and during the course of VFEND therapy. Patients who develop abnormal liver function tests during VFEND therapy should be monitored for the development of more severe hepatic injury. Patient management should include laboratory evaluation of hepatic function (particularly liver function tests and bilirubin). Discontinuation of VFEND must be considered if clinical signs and symptoms consistent with liver disease develop that may be attributable to VFEND.
Visual Disturbances
The effect of VFEND on visual function is not known if treatment continues beyond 28 days. There have been post-marketing reports of prolonged visual adverse events, including optic neuritis and papilledema. If treatment continues beyond 28 days, visual function including visual acuity, visual field and color perception should be monitored.
Embryo-Fetal Toxicity
Voriconazole can cause fetal harm when administered to a pregnant woman.
In animals, voriconazole administration was associated with teratogenicity, embryotoxicity, increased gestational length, dystocia and embryomortality. If this drug is used during pregnancy, or if the patient becomes pregnant while taking this drug, the patient should be informed of the potential hazard to the fetus.
Arrhythmias and QT Prolongation
Some azoles, including voriconazole, have been associated with prolongation of the QT interval on the electrocardiogram. During clinical development and post-marketing surveillance, there have been rare cases of arrhythmias, (including ventricular arrhythmias such as torsade de pointes), cardiac arrests and sudden deaths in patients taking voriconazole. These cases usually involved seriously ill patients with multiple confounding risk factors, such as history of cardiotoxic chemotherapy, cardiomyopathy, hypokalemia and concomitant medications that may have been contributory. Voriconazole should be administered with caution to patients with these potentially proarrhythmic conditions. Rigorous attempts to correct potassium, magnesium and calcium should be made before starting voriconazole.
Infusion Related Reactions
During infusion of the intravenous formulation of voriconazole in healthy subjects, anaphylactoid-type reactions, including flushing, fever, sweating, tachycardia, chest tightness, dyspnea, faintness, nausea, pruritus and rash, have occurred uncommonly. Symptoms appeared immediately upon initiating the infusion. Consideration should be given to stopping the infusion should these reactions occur.
Laboratory Tests
Electrolyte disturbances such as hypokalemia, hypomagnesemia and hypocalcemia should be corrected prior to initiation of VFEND therapy. Patient management should include laboratory evaluation of renal (particularly serum creatinine) and hepatic function (particularly liver function tests and bilirubin).
Patients With Hepatic Impairment
It is recommended that the standard loading dose regimens be used but that the maintenance dose be halved in patients with mild to moderate hepatic cirrhosis (Child-Pugh Class A and B) receiving VFEND [see CLINICAL PHARMACOLOGY (12.3), and DOSAGE AND ADMINISTRATION (2.6)].
VFEND has not been studied in patients with severe cirrhosis (Child-Pugh Class C). VFEND has been associated with elevations in liver function tests and clinical signs of liver damage, such as jaundice, and should only be used in patients with severe hepatic insufficiency if the benefit outweighs the potential risk. Patients with hepatic insufficiency must be carefully monitored for drug toxicity.
Patients With Renal Impairment
In patients with moderate to severe renal dysfunction (creatinine clearance <50 mL/min), accumulation of the intravenous vehicle, SBECD, occurs. Oral voriconazole should be administered to these patients, unless an assessment of the benefit/risk to the patient justifies the use of intravenous voriconazole. Serum creatinine levels should be closely monitored in these patients, and if increases occur, consideration should be given to changing to oral voriconazole therapy.
Monitoring of Renal Function
Acute renal failure has been observed in patients undergoing treatment with VFEND. Patients being treated with voriconazole are likely to be treated concomitantly with nephrotoxic medications and have concurrent conditions that may result in decreased renal function. Patients should be monitored for the development of abnormal renal function. This should include laboratory evaluation, particularly serum creatinine.
Monitoring of Pancreatic Function
Patients with risk factors for acute pancreatitis (e.g., recent chemotherapy, hematopoietic stem cell transplantation [HSCT]) should be monitored for the development of pancreatitis during VFEND treatment.
Dermatological Reactions
Serious exfoliative cutaneous reactions, such as Stevens-Johnson syndrome, have been reported during treatment with VFEND. If a patient develops an exfoliative cutaneous reaction, VFEND should be discontinued. In addition VFEND has been associated with photosensitivity skin reaction. Patients should avoid intense or prolonged exposure to direct sunlight during VFEND treatment. In patients with photosensitivity skin reactions squamous cell carcinoma of the skin and melanoma have been reported during long-term therapy. If a patient develops a skin lesion consistent with squamous cell carcinoma or melanoma, VFEND should be discontinued.
Skeletal Adverse Events
Fluorosis and periostitis have been reported during long-term voriconazole therapy. If a patient develops skeletal pain and radiologic findings compatible with fluorosis or periostitis, voriconazole should be discontinued.
|drugInteractions=
|overdose=In clinical trials, there were three cases of accidental overdose. All occurred in pediatric patients who received up to five times the recommended intravenous dose of voriconazole. A single adverse event of photophobia of 10 minutes duration was reported.
- There is no known antidote to voriconazole.
Voriconazole is hemodialyzed with clearance of 121 mL/min. The intravenous vehicle, SBECD, is hemodialyzed with clearance of 55 mL/min. In an overdose, hemodialysis may assist in the removal of voriconazole and SBECD from the body.
The minimum lethal oral dose in mice and rats was 300 mg/kg (equivalent to 4 and 7 times the recommended maintenance dose (RMD), based on body surface area). At this dose, clinical signs observed in both mice and rats included salivation, mydriasis, titubation (loss of balance while moving), depressed behavior, prostration, partially closed eyes, and dyspnea. Other signs in mice were convulsions, corneal opacification and swollen abdomen. |drugBox=
|mechAction=Voriconazole is an azole antifungal agent. The primary mode of action of voriconazole is the inhibition of fungal cytochrome P-450-mediated 14 alpha-lanosterol demethylation, an essential step in fungal ergosterol biosynthesis. The accumulation of 14 alpha-methyl sterols correlates with the subsequent loss of ergosterol in the fungal cell wall and may be responsible for the antifungal activity of voriconazole. |structure=The structural formula is:
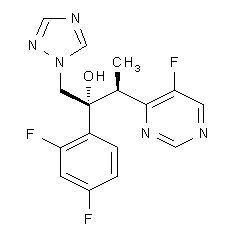
Voriconazole is designated chemically as (2R,3S)-2-(2, 4-difluorophenyl)-3-(5-fluoro-4-pyrimidinyl)-1-(1H-1,2,4-triazol-1-yl)-2-butanol with an empirical formula of C16H14F3N5O and a molecular weight of 349.3. |PK=The pharmacokinetics of voriconazole have been characterized in healthy subjects, special populations and patients.
During oral administration of 200 mg or 300 mg twice daily for 14 days in patients at risk of aspergillosis (mainly patients with malignant neoplasms of lymphatic or hematopoietic tissue), the observed pharmacokinetic characteristics were similar to those observed in healthy subjects.
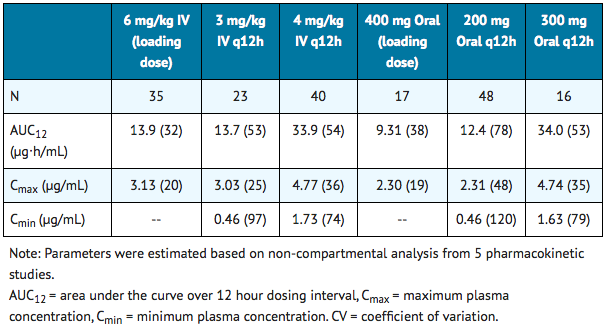
The pharmacokinetics of voriconazole are non-linear due to saturation of its metabolism. The interindividual variability of voriconazole pharmacokinetics is high. Greater than proportional increase in exposure is observed with increasing dose. It is estimated that, on average, increasing the oral dose from 200 mg q12h to 300 mg q12h leads to an approximately 2.5-fold increase in exposure (AUCτ); similarly, increasing the intravenous dose from 3 mg/kg q12h to 4 mg/kg q12h produces an approximately 2.5-fold increase in exposure (Table 9).
Sparse plasma sampling for pharmacokinetics was conducted in the therapeutic studies in patients aged 12–18 years. In 11 adolescent patients who received a mean voriconazole maintenance dose of 4 mg/kg IV, the median of the calculated mean plasma concentrations was 1.60 µg/mL (inter-quartile range 0.28 to 2.73 µg/mL). In 17 adolescent patients for whom mean plasma concentrations were calculated following a mean oral maintenance dose of 200 mg q12h, the median of the calculated mean plasma concentrations was 1.16 µg/mL (inter-quartile range 0.85 to 2.14 µg/mL).
When the recommended intravenous loading dose regimen is administered to healthy subjects, plasma concentrations close to steady state are achieved within the first 24 hours of dosing (eg, 6 mg/kg IV q12h on day 1 followed by 3 mg/kg IV q12h). Without the loading dose, accumulation occurs during twice-daily multiple dosing with steady-state plasma voriconazole concentrations being achieved by day 6 in the majority of subjects.
Absorption
The pharmacokinetic properties of voriconazole are similar following administration by the intravenous and oral routes. Based on a population pharmacokinetic analysis of pooled data in healthy subjects (N=207), the oral bioavailability of voriconazole is estimated to be 96% (CV 13%). Maximum plasma concentrations (Cmax) are achieved 1–2 hours after dosing. When multiple doses of voriconazole are administered with high-fat meals, the mean Cmax and AUCτ are reduced by 34% and 24%, respectively when administered as a tablet and by 58% and 37% respectively when administered as the oral suspension. In healthy subjects, the absorption of voriconazole is not affected by coadministration of oral ranitidine, cimetidine, or omeprazole, drugs that are known to increase gastric pH.
Distribution
The volume of distribution at steady state for voriconazole is estimated to be 4.6 L/kg, suggesting extensive distribution into tissues. Plasma protein binding is estimated to be 58% and was shown to be independent of plasma concentrations achieved following single and multiple oral doses of 200 mg or 300 mg (approximate range: 0.9–15 µg/mL). Varying degrees of hepatic and renal insufficiency do not affect the protein binding of voriconazole.
Metabolism
In vitro studies showed that voriconazole is metabolized by the human hepatic cytochrome P450 enzymes, [[CYP2C19], CYP2C9 and CYP3A4.
|alcohol=Alcohol-Voriconazole interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication. }}
For patient information, click here.
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [2]; Associate Editor(s)-in-Chief: Ahmed Zaghw, M.D. [3]
Overview
Category
Antifungal drug
US Brand Names
Vfend®
FDA Package Insert
Description | Clinical Pharmacology | Microbiology | Indications and Usage | Contraindications | Warnings and Precautions | Adverse Reactions | Drug Interactions | Overdosage | Clinical Studies | Dosage and Administration | How Supplied | Labels and Packages