Voriconazole microbiology
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Ahmed Zaghw, M.D. [2]
Microbiology
Mechanism of Action
Voriconazole is a triazole antifungal agent. The primary mode of action of voriconazole is the inhibition of fungal cytochrome P-450-mediated 14 alpha-lanosterol demethylation, an essential step in fungal ergosterol biosynthesis. The accumulation of 14 alpha-methyl sterols correlates with the subsequent loss of ergosterol in the fungal cell wall and may be responsible for the antifungal activity of voriconazole.
Activity In Vitro and In Vivo
Voriconazole has been shown to be active against most strains of the following microorganisms, both in vitro and in clinical infections.
- Aspergillus fumigatus
- Aspergillus flavus
- Aspergillus niger
- Aspergillus terreus
- Candida albicans
- Candida glabrata (In clinical studies, the voriconazole MIC90 was 4 µg/mL) ‡
- Candida krusei
- Candida parapsilosis
- Candida tropicalis
- Fusarium spp. including Fusarium solani
- Scedosporium apiospermum
The following data are available, but their clinical significance is unknown.
Voriconazole exhibits in vitro minimal inhibitory concentrations (MICs) of 1 µg/mL or less against most (≥90%) isolates of the following microorganisms; however, the safety and effectiveness of voriconazole in treating clinical infections due to these Candida species have not been established in adequate and well-controlled clinical trials:
- Candida lusitaniae
- Candida guilliermondii
‡ In clinical studies, voriconazole MIC90 for C. glabrata baseline isolates was 4 µg/mL; 13/50 (26%) C. glabrata baseline isolates were resistant (MIC ≥4 µg/mL) to voriconazole. However, based on 1054 isolates tested in surveillance studies the MIC90 was 1 µg/mL (see Table 12).
Susceptibility Testing Methods
Aspergillus species and other filamentous fungi
No interpretive criteria have been established for Aspergillus species and other filamentous fungi.
Candida species
The interpretive standards for voriconazole against Candida species are applicable only to tests performed using Clinical Laboratory and Standards Institute (CLSI) microbroth dilution reference method M27 for MIC read at 48 hours or disk diffusion reference method M44 for zone diameter read at 24 hours.2,3
Broth Microdilution Techniques–Quantitative methods are used to determine antifungal minimum inhibitory concentrations (MICs). These MICs provide estimates of the susceptibility of Candida spp. to antifungal agents. MICs should be determined using a standardized procedure at 48 hours.2 Standardized procedures are based on a microdilution method (broth) with standardized inoculum concentrations and standardized concentrations of voriconazole powder. The MIC values should be interpreted according to the criteria provided in Table 12.
Diffusion Techniques–Qualitative methods that require measurement of zone diameters also provide reproducible estimates of the susceptibility of Candidaspp. to an antifungal agent. One such standardized procedure requires the use of standardized inoculum concentrations.3 This procedure uses paper disks impregnated with 1 µg of voriconazole to test the susceptibility of yeasts to voriconazole at 24 hours. Disk diffusion interpretive criteria are also provided in Table 12.
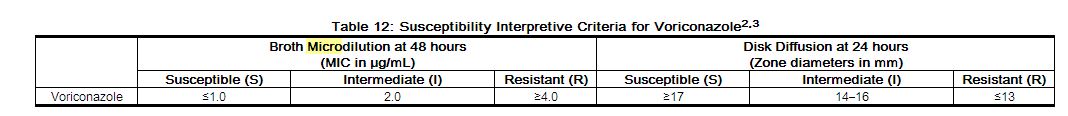 |
NOTE: Shown are the breakpoints (µg/mL) for voriconazole against Candida species.
The susceptible category implies that isolates are inhibited by the usually achievable concentrations of antifungal agent tested when the recommended dosage is used for the site of infection. The intermediate category implies that an infection due to the isolate may be appropriately treated in body sites where the drugs are physiologically concentrated or when a high dosage of drug is used. The resistant category implies that isolates are not inhibited by the usually achievable concentrations of the agent with normal dosage schedules and clinical efficacy of the agent against the isolate has not been reliably shown in treatment studies.
Quality Control
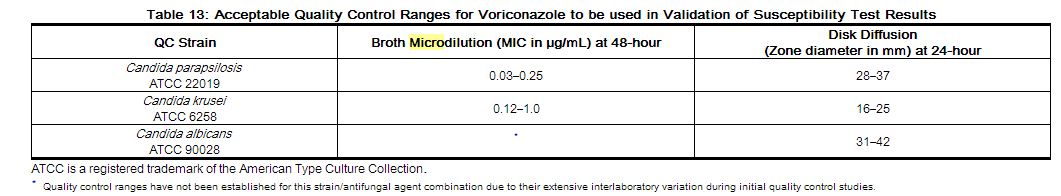
Standardized susceptibility test procedures require the use of quality control organisms to ensure the accuracy of the technical aspects of the test procedures. Standard voriconazole powder and 1 µg disks should provide the following range of values noted in Table 13.
NOTE: Quality control microorganisms are specific strains of organisms with intrinsic biological properties relating to resistance mechanisms and their genetic expression within fungi; the specific strains used for microbiological control are not clinically significant.
 |
Activity In Animal Models
Voriconazole was active in normal and/or immunocompromised guinea pigs with systemic and/or pulmonary infections due to A. fumigatus (including an isolate with reduced susceptibility to itraconazole) or Candida species [C. albicans (including an isolate with reduced susceptibility to fluconazole), C. krusei and C. glabrata] in which the endpoints were prolonged survival of infected animals and/or reduction of mycological burden from target organs. In one experiment, voriconazole exhibited activity against Scedosporium apiospermum infections in immune competent guinea pigs.
Drug Resistance
Voriconazole drug resistance development has not been adequately studied in vitro against Candida, Aspergillus, Scedosporium and Fusarium species. The frequency of drug resistance development for the various fungi for which this drug is indicated is not known.
Fungal isolates exhibiting reduced susceptibility to fluconazole or itraconazole may also show reduced susceptibility to voriconazole, suggesting cross-resistance can occur among these azoles. The relevance of cross-resistance and clinical outcome has not been fully characterized. Clinical cases where azole cross-resistance is demonstrated may require alternative antifungal therapy. [1]
References
Adapted from the FDA Package Insert.