Rifaximin
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Vignesh Ponnusamy, M.B.B.S. [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Rifaximin is a rifamycin antibacterial that is FDA approved for the treatment of travelers’ diarrhea (TD) caused by noninvasive strains of Escherichia coli and reduction in risk of overt hepatic encephalopathy (HE) recurrence in patients ≥ 18 years of age. Common adverse reactions include flatulence, headache, abdominal pain, rectal tenesmus, defecation urgency, nausea, peripheral edema, dizziness, fatigue, and ascites.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Travelers’ Diarrhea
- Dosing Information
- The recommended dose of XIFAXAN is one 200 mg tablet taken orally three times a day for 3 days. XIFAXAN can be administered orally, with or without food.
Hepatic Encephalopathy
- Dosing Information
- The recommended dose of XIFAXAN is one 550 mg tablet taken orally two times a day, with or without food.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Rifaximin in adult patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Rifaximin in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
Travelers’ Diarrhea
- Dosing Information
- The recommended dose of XIFAXAN is one 200 mg tablet taken orally three times a day for 3 days (12 years or older). XIFAXAN can be administered orally, with or without food.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Rifaximin in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Rifaximin in pediatric patients.
Contraindications
- Hypersensitivity
- XIFAXAN is contraindicated in patients with a hypersensitivity to rifaximin, any of the rifamycin antimicrobial agents, or any of the components in XIFAXAN. Hypersensitivity reactions have included exfoliative dermatitis, angioneurotic edema, and anaphylaxis.
Warnings
Precautions
- Travelers’ Diarrhea Not Caused by Escherichia coli
- XIFAXAN was not found to be effective in patients with diarrhea complicated by fever and/or blood in the stool or diarrhea due to pathogens other than Escherichia coli.
- Discontinue XIFAXAN if diarrhea symptoms get worse or persist more than 24-48 hours and alternative antibiotic therapy should be considered.
- XIFAXAN is not effective in cases of travelers’ diarrhea. The effectiveness of XIFAXAN in travelers’ diarrhea caused by spp. and spp. has not been proven. XIFAXAN should not be used in patients where , spp., or spp. may be suspected as causative pathogens.
- Clostridium difficile-Associated Diarrhea
- Clostridium difficile-associated diarrhea (CDAD) has been reported with use of nearly all antibacterial agents, including XIFAXAN, and may range in severity from mild diarrhea to fatal colitis. Treatment with antibacterial agents alters the normal flora of the colon which may lead to overgrowth of Clostridium difficile. C. difficile produces toxins A and B which contribute to the development of CDAD. Hypertoxin producing strains of cause increased morbidity and mortality, as these infections can be refractory to antimicrobial therapy and may require colectomy. CDAD must be considered in all patients who present with diarrhea following antibiotic use. Careful medical history is necessary since CDAD has been reported to occur over two months after the administration of antibacterial agents.
- If CDAD is suspected or confirmed, ongoing antibiotic use not directed against may need to be discontinued. Appropriate fluid and electrolyte management, protein supplementation, antibiotic treatment of , and surgical evaluation should be instituted as clinically indicated.
- Development of Drug Resistant Bacteria
- Prescribing XIFAXAN for travelers’ diarrhea in the absence of a proven or strongly suspected bacterial infection or a prophylactic indication is unlikely to provide benefit to the patient and increases the risk of the development of drug-resistant bacteria.
- Severe (Child-Pugh C) Hepatic Impairment
- There is increased systemic exposure in patients with severe hepatic impairment. Animal toxicity studies did not achieve systemic exposures that were seen in patients with severe hepatic impairment. The clinical trials were limited to patients with MELD scores <25. Therefore, caution should be exercised when administering XIFAXAN to patients with severe hepatic impairment (Child-Pugh C).
- Concomitant use with P-glycoprotein Inhibitors
- Concomitant administration of drugs that are P-glycoprotein inhibitors with rifaximin can substantially increase the systemic exposure to rifaximin. Caution should be exercised when concomitant use of rifaximin and a P-glycoprotein inhibitor such as cyclosporine is needed. In patients with hepatic impairment, a potential additive effect of reduced metabolism and concomitant P-glycoprotein inhibitors may further increase the systemic exposure to rifaximin.
Adverse Reactions
Clinical Trials Experience
- Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
Travelers’ Diarrhea
- The safety of XIFAXAN 200 mg taken three times a day was evaluated in patients with travelers’ diarrhea consisting of 320 patients in two placebo-controlled clinical trials with 95% of patients receiving three or four days of treatment with XIFAXAN. The population studied had a mean age of 31.3 (18-79) years of which approximately 3% were ≥ 65 years old, 53% were male and 84% were White, 11% were Hispanic.
- Discontinuations due to adverse reactions occurred in 0.4% of patients. The adverse reactions leading to discontinuation were taste loss, dysentery, weight loss, anorexia, nausea and nasal passage irritation.
- All adverse reactions for XIFAXAN 200 mg three times daily that occurred at a frequency ≥ 2% in the two placebo-controlled trials combined are provided in Table 1. (These include adverse reactions that may be attributable to the underlying disease.)
- The following adverse reactions, presented by body system, have also been reported in <2% of patients taking XIFAXAN in the two placebo-controlled clinical trials where the 200 mg tablet was taken three times a day for travelers’ diarrhea. The following includes adverse reactions regardless of causal relationship to drug exposure.
Blood and Lymphatic System Disorders
Lymphocytosis, monocytosis, neutropenia
Ear and Labyrinth Disorders
Ear pain, motion sickness, tinnitus
Gastrointestinal Disorders
Abdominal distension, diarrhea NOS, dry throat, fecal abnormality NOS, gingival disorder NOS, inguinal hernia NOS, dry lips, stomach discomfort
General Disorders and Administration Site Conditions
Chest pain, fatigue, malaise, pain NOS, weakness
Infections and Infestations
Dysentery NOS, respiratory tract infection NOS, upper respiratory tract infection NOS
Investigations
Aspartate aminotransferase increased, blood in stool, blood in urine, weight decreased
Metabolic and Nutritional Disorders
Musculoskeletal, Connective Tissue, and Bone Disorders
Arthralgia, muscle spasms, myalgia, neck pain
Nervous System Disorders
Abnormal dreams, dizziness, migraine NOS, syncope, loss of taste
Psychiatric Disorders
Renal and Urinary Disorders
Choluria, dysuria, hematuria, polyuria, proteinuria, urinary frequency
Respiratory, Thoracic, and Mediastinal Disorders
Dyspnea NOS, nasal passage irritation, nasopharyngitis, pharyngitis, pharyngolaryngeal pain, rhinitis NOS, rhinorrhea
Skin and Subcutaneous Tissue Disorders
Clamminess, rash NOS, sweating increased
Vascular Disorders
Hot flashes NOS
Hepatic Encephalopathy
- The data described below reflect exposure to XIFAXAN 550 mg in 348 patients, including 265 exposed for 6 months and 202 exposed for more than a year (mean exposure was 364 days). The safety of XIFAXAN 550 mg taken two times a day for reducing the risk of overt hepatic encephalopathy recurrence in adult patients was evaluated in a 6-month placebo-controlled clinical trial (n = 140) and in a long term follow-up study (n = 280). The population studied had a mean age of 56.26 (range: 21-82) years; approximately 20% of the patients were ≥ 65 years old, 61% were male, 86% were White, and 4% were Black. Ninety-one percent of patients in the trial were taking lactulose concomitantly. All adverse reactions that occurred at an incidence ≥ 5% and at a higher incidence in XIFAXAN 550 mg-treated subjects than in the placebo group in the 6-month trial are provided in Table 2. (These include adverse events that may be attributable to the underlying disease).
- The following adverse reactions, presented by body system, have also been reported in the placebo-controlled clinical trial in greater than 2% but less than 5% of patients taking XIFAXAN 550 mg taken orally two times a day for hepatic encephalopathy. The following includes adverse events occurring at a greater incidence than placebo, regardless of causal relationship to drug exposure.
Ear and Labyrinth Disorders
Gastrointestinal Disorders
Lower abdominal pain, abdominal tenderness, dry mouth, esophageal variceal bleed, stomach discomfort
General Disorders and Administration Site Conditions
Chest pain, generalized edema, influenza like illness, pain NOS
Infections and Infestations
Cellulitis, pneumonia, rhinitis, upper respiratory tract infection NOS
Poisoning and Procedural Complications
Contusion, fall, procedural pain Injury
Metabolic and Nutritional Disorders
Anorexia, dehydration, hyperglycemia, hyperkalemia, hypoglycemia, hyponatremia
Musculoskeletal, Connective Tissue, and Bone Disorders
Myalgia, pain in extremity
Nervous System Disorders
Amnesia, disturbance in attention, hypoesthesia, memory impairment, tremor
Psychiatric Disorders
Confusional state
Respiratory, Thoracic, and Mediastinal Disorders
Vascular Disorders
Postmarketing Experience
- The following adverse reactions have been identified during post approval use of XIFAXAN. Because these reactions are reported voluntarily from a population of unknown size, estimates of frequency cannot be made. These reactions have been chosen for inclusion due to either their seriousness, frequency of reporting or causal connection to XIFAXAN.
Infections and Infestations
Cases of C. difficile-associated colitis have been reported.
General
Hypersensitivity reactions, including exfoliative dermatitis, rash, angioneurotic edema (swelling of face and tongue and difficulty swallowing), urticaria, flushing, pruritus and anaphylaxis have been reported. These events occurred as early as within 15 minutes of drug administration.
Drug Interactions
- Effects of rifaximin on cytochrome P450 enzymes
- studies have shown that rifaximin did not inhibit cytochrome P450 isoenzymes 1A2, 2A6, 2B6, 2C9, 2C19, 2D6, 2E1 and CYP3A4 at concentrations ranging from 2 to 200 ng/mL. Rifaximin is not expected to inhibit these enzymes in clinical use.
- An study has suggested that rifaximin induces CYP3A4. However, in patients with normal liver function, rifaximin at the recommended dosing regimen is not expected to induce CYP3A4. It is unknown whether rifaximin can have a significant effect on the pharmacokinetics of concomitant CYP3A4 substrates in patients with reduced liver function who have elevated rifaximin concentrations.
- Concomitant P-glycoprotein inhibitors
- An study suggested that rifaximin is a substrate of P-glycoprotein. Co-administration of cyclosporine, a potent P-glycoprotein inhibitor, with rifaximin resulted in 83-fold and 124-fold increases in rifaximin mean C and AUC∞ in healthy subjects. The clinical significance of this increase in systemic exposure is unknown.
Use in Specific Populations
Pregnancy
- Pregnancy Category C
- There are no adequate and well controlled studies in pregnant women. Rifaximin has been shown to be teratogenic in rats and rabbits at doses that caused maternal toxicity. XIFAXAN tablets should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
- Administration of rifaximin to pregnant rats and rabbits at dose levels that caused reduced body weight gain resulted in eye malformations in both rat and rabbit fetuses. Additional malformations were observed in fetal rabbits that included cleft palate, lumbar scoliosis, brachygnathia, interventricular septal defect, and large atrium.
- The fetal rat malformations were observed in a study of pregnant rats administered a high dose that resulted in 16 times the therapeutic dose to diarrheic patients or 1 times the therapeutic dose to patients with hepatic encephalopathy (based upon plasma AUC comparisons). Fetal rabbit malformations were observed from pregnant rabbits administered mid and high doses that resulted in 1 or 2 times the therapeutic dose to diarrheic patients or less than 0.1 times the dose in patients with hepatic encephalopathy, based upon plasma AUC comparisons.
- Post-natal developmental effects were not observed in rat pups from pregnant/lactating female rats dosed during the period from gestation to Day 20 post-partum at the highest dose which resulted in approximately 16 times the human therapeutic dose for travelers’ diarrhea (based upon AUCs) or approximately 1 times the AUCs derived from therapeutic doses to patients with hepatic encephalopathy.
- Australian Drug Evaluation Committee (ADEC) Pregnancy Category
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Rifaximin in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Rifaximin during labor and delivery.
Nursing Mothers
- It is not known whether rifaximin is excreted in human milk. Because many drugs are excreted in human milk and because of the potential for adverse reactions in nursing infants from XIFAXAN, a decision should be made whether to discontinue nursing or to discontinue the drug, taking into account the importance of the drug to the mother.
Pediatric Use
- The safety and effectiveness of XIFAXAN 200 mg in pediatric patients with travelers’ diarrhea less than 12 years of age have not been established.
- The safety and effectiveness of XIFAXAN 550 mg for HE have not been established in patients < 18 years of age.
Geriatic Use
- Clinical studies with rifaximin 200 mg for travelers’ diarrhea did not include sufficient numbers of patients aged 65 and over to determine whether they respond differently than younger subjects.
- In the controlled trial with XIFAXAN 550 mg for hepatic encephalopathy, 19.4% were 65 and over, while 2.3% were 75 and over. No overall differences in safety or effectiveness were observed between these subjects and younger subjects, and other reported clinical experience has not identified differences in responses between the elderly and younger patients, but greater sensitivity of some older individuals cannot be ruled out.
Gender
There is no FDA guidance on the use of Rifaximin with respect to specific gender populations.
Race
There is no FDA guidance on the use of Rifaximin with respect to specific racial populations.
Renal Impairment
- The pharmacokinetics of rifaximin in patients with impaired renal function has not been studied.
Hepatic Impairment
- Following administration of XIFAXAN 550 mg twice daily to patients with a history of hepatic encephalopathy, the systemic exposure (i.e., AUC ) of rifaximin was about 10-, 13-, and 20-fold higher in those patients with mild (Child-Pugh A), moderate (Child-Pugh B) and severe (Child-Pugh C) hepatic impairment, respectively, compared to that in healthy volunteers. No dosage adjustment is recommended because rifaximin is presumably acting locally. Nonetheless, caution should be exercised when XIFAXAN is administered to patients with severe hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Rifaximin in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Rifaximin in patients who are immunocompromised.
Administration and Monitoring
Administration
- Oral
Monitoring
There is limited information regarding Monitoring of Rifaximin in the drug label.
IV Compatibility
There is limited information regarding IV Compatibility of Rifaximin in the drug label.
Overdosage
Acute Overdose
- No specific information is available on the treatment of overdosage with XIFAXAN. In clinical studies at doses higher than the recommended dose (> 600 mg/day for travelers’ diarrhea or > 1100 mg/day for hepatic encephalopathy), adverse reactions were similar in subjects who received doses higher than the recommended dose and placebo. In the case of overdosage, discontinue XIFAXAN, treat symptomatically, and institute supportive measures as required.
Chronic Overdose
There is limited information regarding Chronic Overdose of Rifaximin in the drug label.
Pharmacology
Mechanism of Action
- Rifaximin is a non-aminoglycoside semi-synthetic antibacterial derived from rifamycin SV. Rifaximin acts by binding to the beta-subunit of bacterial DNA-dependent RNA polymerase resulting in inhibition of bacterial RNA synthesis.
Structure
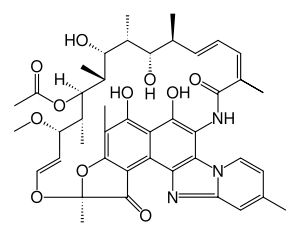
- XIFAXAN tablets contain rifaximin, a non-aminoglycoside semi-synthetic, nonsystemic antibiotic derived from rifamycin SV. Rifaximin is a structural analog of rifampin. The chemical name for rifaximin is (2 ,16 ,18 ,20 ,21 ,22 ,23 ,24 ,25 ,26 ,27 ,28 )-5,6,21,23,25-pentahydroxy-27-methoxy-2,4,11,16,20,22,24,26-octamethyl-2,7-(epoxypentadeca-[1,11,13]trienimino)benzofuro[4,5-e]pyrido[1,2-á]-benzimidazole-1,15(2 )-dione,25-acetate. The empirical formula is C H N O and its molecular weight is 785.9. The chemical structure is represented below: SZESSRRRSSSEH4351311
- XIFAXAN tablets for oral administration are film-coated and contain 200 mg or 550 mg of rifaximin.
- Inactive ingredients:
- Each 200 mg tablet contains colloidal silicon dioxide, disodium edetate, glycerol palmitostearate, hypromellose, microcrystalline cellulose, propylene glycol, red iron oxide, sodium starch glycolate, talc, and titanium dioxide.
- Each 550 mg tablet contains colloidal silicon dioxide, glycerol palmitostearate, microcrystalline cellulose, polyethylene glycol/macrogol, polyvinyl alcohol, red iron oxide, sodium starch glycolate, talc, and titanium dioxide.
Pharmacodynamics
There is limited information regarding Pharmacodynamics of Rifaximin in the drug label.
Pharmacokinetics
- Absorption
- Travelers’ Diarrhea
- Systemic absorption of rifaximin (200 mg three times daily) was evaluated in 13 subjects challenged with shigellosis on Days 1 and 3 of a three-day course of treatment. Rifaximin plasma concentrations and exposures were low and variable. There was no evidence of accumulation of rifaximin following repeated administration for 3 days (9 doses). Peak plasma rifaximin concentrations after 3 and 9 consecutive doses ranged from 0.81 to 3.4 ng/mL on Day 1 and 0.68 to 2.26 ng/mL on Day 3. Similarly, AUC estimates were 6.95 ± 5.15 ng•h/mL on Day 1 and 7.83 ± 4.94 ng•h/mL on Day 3. XIFAXAN is not suitable for treating systemic bacterial infections because of limited systemic exposure after oral administration .
- Hepatic Encephalopathy
- After a single dose and multiple doses of rifaximin 550 mg in healthy subjects, the mean time to reach peak plasma concentrations was about an hour. The pharmacokinetic (PK) parameters were highly variable and the accumulation ratio based on AUC was 1.37.
- The PK of rifaximin in patients with a history of HE was evaluated after administration of XIFAXAN, 550 mg two times a day. The PK parameters were associated with a high variability and mean rifaximin exposure (AUC ) in patients with a history of HE (147 ng•h/mL) was approximately 12-fold higher than that observed in healthy subjects following the same dosing regimen (12.3 ng•h/mL). When PK parameters were analyzed based on Child-Pugh Class A, B, and C, the mean AUC was 10-, 13-, and 20-fold higher, respectively, compared to that in healthy subjects (Table 3).
- Food Effect in Healthy Subjects
- A high-fat meal consumed 30 minutes prior to XIFAXAN dosing in healthy subjects delayed the mean time to peak plasma concentration from 0.75 to 1.5 hours and increased the systemic exposure (AUC) of rifaximin by 2-fold (Table 4).
- Distribution
- Rifaximin is moderately bound to human plasma proteins, the mean protein binding ratio was 67.5% in healthy subjects and 62% in patients with hepatic impairment when XIFAXAN 550 mg was administered.
- Metabolism and Excretion
- In a mass balance study, after administration of 400 mg C-rifaximin orally to healthy volunteers, of the 96.94% total recovery, 96.62% of the administered radioactivity was recovered in feces almost exclusively as the unchanged drug and 0.32% was recovered in urine mostly as metabolites with 0.03% as the unchanged drug. Rifaximin accounted for 18% of radioactivity in plasma. This suggests that the absorbed rifaximin undergoes metabolism with minimal renal excretion of the unchanged drug. The enzymes responsible for metabolizing rifaximin are unknown.
- In a separate study, rifaximin was detected in the bile after cholecystectomy in patients with intact gastrointestinal mucosa, suggesting biliary excretion of rifaximin.
- Specific Populations
- Hepatic Impairment
- The systemic exposure of rifaximin was markedly elevated in patients with hepatic impairment compared to healthy subjects. The mean AUC in patients with Child-Pugh Class C hepatic impairment was 2-fold higher than in patients with Child-Pugh Class A hepatic impairment (see Table 3).
- Renal Impairment
- The pharmacokinetics of rifaximin in patients with impaired renal function has not been studied.
- Drug Interactions
- Midazolam
- The effect of rifaximin 200 mg administered orally every 8 hours for 3 days and for 7 days on the pharmacokinetics of a single dose of either midazolam 2 mg intravenous or midazolam 6 mg orally was evaluated in healthy subjects. No significant difference was observed in the metrics of systemic exposure or elimination of intravenous or oral midazolam or its major metabolite, 1’-hydroxymidazolam, between midazolam alone or together with rifaximin. Therefore, rifaximin was not shown to significantly affect intestinal or hepatic CYP3A4 activity for the 200 mg three times a day dosing regimen.
- After XIFAXAN 550 mg was administered three times a day for 7 days and 14 days to healthy subjects, the mean AUC of single midazolam 2 mg orally was 3.8% and 8.8% lower, respectively, than when midazolam was administered alone. The mean C of midazolam was also decreased by 4-5% when XIFAXAN was administered for 7-14 days prior to midazolam administration. This degree of interaction is not considered clinically meaningful. max
- The effect of rifaximin on CYP3A4 in patients with impaired liver function who have elevated systemic exposure is not known.
- Oral Contraceptives Containing 0.07 mg Ethinyl Estradiol and 0.5 mg Norgestimate
- The oral contraceptive study utilized an open-label, crossover design in 28 healthy female subjects to determine if rifaximin 200 mg orally administered three times a day for 3 days (the dosing regimen for travelers’ diarrhea) altered the pharmacokinetics of a single dose of an oral contraceptive containing 0.07 mg ethinyl estradiol and 0.5 mg norgestimate. Results showed that the pharmacokinetics of single doses of ethinyl estradiol and norgestimate were not altered by rifaximin.
- Effect of rifaximin on oral contraceptives was not studied for XIFAXAN 550 mg twice a day, the dosing regimen for hepatic encephalopathy.
Nonclinical Toxicology
- Malignant schwannomas in the heart were significantly increased in male Crl:CD® (SD) rats that received rifaximin by oral gavage for two years at 150 to 250 mg/kg/day (doses equivalent to 2.4 to 4 times the recommended dose of 200 mg three times daily for travelers’ diarrhea, and equivalent to 1.3 to 2.2 times the recommended dose of 550 mg twice daily for hepatic encephalopathy, based on relative body surface area comparisons). There was no increase in tumors in Tg.rasH2 mice dosed orally with rifaximin for 26 weeks at 150 to 2000 mg/kg/day (doses equivalent to 1.2 to 16 times the recommended daily dose for travelers’ diarrhea and equivalent to 0.7 to 9 times the recommended daily dose for hepatic encephalopathy, based on relative body surface area comparisons).
- Rifaximin was not genotoxic in the bacterial reverse mutation assay, chromosomal aberration assay, rat bone marrow micronucleus assay, rat hepatocyte unscheduled DNA synthesis assay, or the CHO/HGPRT mutation assay. There was no effect on fertility in male or female rats following the administration of rifaximin at doses up to 300 mg/kg (approximately 5 times the clinical dose of 600 mg/day, and approximately 2.6 times the clinical dose of 1100 mg/day, adjusted for body surface area).
Clinical Studies
Travelers’ Diarrhea
- The efficacy of XIFAXAN given as 200 mg orally taken three times a day for 3 days was evaluated in 2 randomized, multi‑center, double-blind, placebo-controlled studies in adult subjects with travelers’ diarrhea. One study was conducted at clinical sites in Mexico, Guatemala, and Kenya (Study 1). The other study was conducted in Mexico, Guatemala, Peru, and India (Study 2). Stool specimens were collected before treatment and 1 to 3 days following the end of treatment to identify enteric pathogens.
- The clinical efficacy of XIFAXAN was assessed by the time to return to normal, formed stools and resolution of symptoms. The primary efficacy endpoint was time to last unformed stool (TLUS) which was defined as the time to the last unformed stool passed, after which clinical cure was declared. Table 5 displays the median TLUS and the number of patients who achieved clinical cure for the intent to treat (ITT) population of Study 1. The duration of diarrhea was significantly shorter in patients treated with XIFAXAN than in the placebo group. More patients treated with XIFAXAN were classified as clinical cures than were those in the placebo group.
- Microbiological eradication (defined as the absence of a baseline pathogen in culture of stool after 72 hours of therapy) rates for Study 1 are presented in Table 6 for patients with any pathogen at baseline and for the subset of patients with at baseline.
- Even though XIFAXAN had microbiologic activity similar to placebo, it demonstrated a clinically significant reduction in duration of diarrhea and a higher clinical cure rate than placebo. Therefore, patients should be managed based on clinical response to therapy rather than microbiologic response.
- The results of Study 2 supported the results presented for Study 1. In addition, this study provided evidence that subjects treated with XIFAXAN with fever and/or blood in the stool at baseline had prolonged TLUS. These subjects had lower clinical cure rates than those without fever or blood in the stool at baseline. Many of the patients with fever and/or blood in the stool (dysentery-like diarrheal syndromes) had invasive pathogens, primarily ,isolated in the baseline stool.
- Also in this study, the majority of the subjects treated with XIFAXAN who had isolated as a sole pathogen at baseline failed treatment and the resulting clinical cure rate for these patients was 23.5% (4/17). In addition to not being different from placebo, the microbiologic eradication rates for subjects with isolated at baseline were much lower than the eradication rates seen.
- In an unrelated open-label, pharmacokinetic study of oral XIFAXAN 200 mg taken every 8 hours for 3 days, 15 adult subjects were challenged with 2a, of whom 13 developed diarrhea or dysentery and were treated with XIFAXAN. Although this open-label challenge trial was not adequate to assess the effectiveness of XIFAXAN in the treatment of shigellosis, the following observations were noted: eight subjects received rescue treatment with ciprofloxacin either because of lack of response to XIFAXAN treatment within 24 hours, or because they developed severe dysentery, or because of recurrence of in the stool; five of the 13 subjects received ciprofloxacin although they did not have evidence of severe disease or relapse.
Hepatic Encephalopathy
- The efficacy of XIFAXAN 550 mg taken orally two times a day was evaluated in a randomized, placebo-controlled, double-blind, multi-center 6-month trial of adult subjects from the U.S., Canada and Russia who were defined as being in remission (Conn score of 0 or 1) from hepatic encephalopathy (HE). Eligible subjects had ³ 2 episodes of HE associated with chronic liver disease in the previous 6 months.
- A total of 299 subjects were randomized to receive either XIFAXAN (n=140) or placebo (n=159) in this study. Patients had a mean age of 56 years (range, 21-82 years), 81% < 65 years of age, 61% were male and 86% White. At baseline, 67% of patients had a Conn score of 0 and 68% had an asterixis grade of 0. Patients had MELD scores of either £ 10 (27%) or 11 to 18 (64%) at baseline. No patients were enrolled with a MELD score of > 25. Nine percent of the patients were Child-Pugh Class C. Lactulose was concomitantly used by 91% of the patients in each treatment arm of the study. Per the study protocol, patients were withdrawn from the study after experiencing a breakthrough HE episode. Other reasons for early study discontinuation included: adverse reactions (XIFAXAN 6%; placebo 4%), patient request to withdraw (XIFAXAN 4%; placebo 6%) and other (XIFAXAN 7%; placebo 5%).
- The primary endpoint was the time to first breakthrough overt HE episode. A breakthrough overt HE episode was defined as a marked deterioration in neurological function and an increase of Conn score to Grade ≥ 2. In patients with a baseline Conn score of 0, a breakthrough overt HE episode was defined as an increase in Conn score of 1 and asterixis grade of 1.
- Breakthrough overt HE episodes were experienced by 31 of 140 subjects (22%) in the XIFAXAN group and by 73 of 159 subjects (46%) in the placebo group during the 6-month treatment period. Comparison of Kaplan-Meier estimates of event-free curves showed XIFAXAN significantly reduced the risk of HE breakthrough by 58% during the 6-month treatment period. Presented below in Figure 1 is the Kaplan-Meier event-free curve for all subjects (n = 299) in the study.
- Event-free refers to non-occurrence of breakthrough HE.
- When the results were evaluated by the following demographic and baseline characteristics, the treatment effect of XIFAXAN 550 mg in reducing the risk of breakthrough overt HE recurrence was consistent for: sex, baseline Conn score, duration of current remission and diabetes. The differences in treatment effect could not be assessed in the following subpopulations due to small sample size: non-White (n=42), baseline MELD > 19 (n=26), Child-Pugh C (n=31), and those without concomitant lactulose use (n=26).
- HE-related hospitalizations (hospitalizations directly resulting from HE, or hospitalizations complicated by HE) were reported for 19 of 140 subjects (14%) and 36 of 159 subjects (23%) in the XIFAXAN and placebo groups respectively. Comparison of Kaplan-Meier estimates of event-free curves showed XIFAXAN significantly reduced the risk of HE-related hospitalizations by 50% during the 6-month treatment period. Comparison of Kaplan-Meier estimates of event-free curves is shown in Figure 2.
How Supplied
- NDC:68151-4980-0 in a PACKAGE of 1 TABLETS
- The 200 mg tablet is a pink-colored, round, biconvex tablet with “Sx” debossed on one side. It is available in the following presentations:
- NDC 65649-301-03, bottles of 30 tablets
- NDC 65649-301-41, bottles of 100 tablets
- NDC 65649-301-05, carton of 100 tablets, Unit Dose
- The 550 mg tablet is a pink-colored, oval, biconvex tablet with “rfx” debossed on one side. It is available in the following presentations:
- NDC 65649-303-02, bottles of 60 tablets
- NDC 65649-303-03, carton of 60 tablets, Unit Dose
- Storage
- Store XIFAXAN Tablets at 20–25°C (68–77°F); excursions permitted to 15–30°C (59-86°F).
Storage
There is limited information regarding Rifaximin Storage in the drug label.
Images
Drug Images
{{#ask: Page Name::Rifaximin |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
{{#ask: Label Page::Rifaximin |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
- Persistent Diarrhea
- Clostridium difficile-Associated Diarrhea
- Clostridium difficile-associated diarrhea (CDAD) has been reported with use of nearly all antibacterial agents, including XIFAXAN, and may range in severity from mild diarrhea to fatal colitis. Treatment with antibiotics alters the normal flora of the colon which may lead to. Patients can develop watery and bloody stools (with or without stomach cramps and fever) even as late as two or more months after having taken the last dose of the antibiotic. If diarrhea occurs after therapy or does not improve or worsens during therapy, advise patients to contact a physician as soon as possible.
- Administration with Food
- Inform patients that XIFAXAN may be taken with or without food.
- Antibacterial Resistance
- Counsel patients that antibacterial drugs including XIFAXAN should only be used to treat bacterial infections. They do not treat viral infections (e.g., the common cold). When XIFAXAN is prescribed to treat a bacterial infection, patients should be told that although it is common to feel better early in the course of therapy, the medication should be taken exactly as directed. Skipping doses or not completing the full course of therapy may (1) decrease the effectiveness of the immediate treatment and (2) increase the likelihood that bacteria will develop resistance and will not be treatable by XIFAXAN or other antibacterial drugs in the future.
- Severe Hepatic Impairment
- Patients should be informed that in patients with severe hepatic impairment (Child-Pugh C) there is an increase in systemic exposure to XIFAXAN.
Precautions with Alcohol
- Alcohol-Rifaximin interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
- XIFAXAN®[1]
Look-Alike Drug Names
- rifaximin® — rifampin®[2]
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
- ↑ "XIFAXAN rifaximin tablet".
- ↑ "http://www.ismp.org". External link in
|title=(help)
{{#subobject:
|Page Name=Rifaximin
|Pill Name=No image.jpg
|Drug Name=
|Pill Ingred=|+sep=;
|Pill Imprint=
|Pill Dosage={{{dosageValue}}} {{{dosageUnit}}}
|Pill Color=|+sep=;
|Pill Shape=
|Pill Size (mm)=
|Pill Scoring=
|Pill Image=
|Drug Author=
|NDC=
}}
{{#subobject:
|Label Page=Rifaximin |Label Name=Rifaximin07.png
}}
{{#subobject:
|Label Page=Rifaximin |Label Name=Rifaximin08.png
}}