Norgestimate and Ethinyl estradiol
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Deepika Beereddy, MBBS [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Black Box Warning
|
WARNINGS: CARDIOVASCULAR RISK ASSOCIATED WITH SMOKING
See full prescribing information for complete Boxed Warning.
Cardiovascular risk associated with smoking:
|
Overview
Norgestimate and Ethinyl estradiol is a contraceptive agent that is FDA approved for the treatment of acne and contraception. There is a Black Box Warning for this drug as shown here. Common adverse reactions include edema, chloasma, weight change finding, bloating symptom, nausea, stomach cramps, vomiting, depression, amenorrhea, break-through bleeding, breast tenderness, discharge from the breast, disorder of menstruation, infertility, temporary after discontinuation of treatment, scanty vaginal bleeding, swelling of breast.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Contraception
- MonoNessa® is indicated for the prevention of pregnancy in women who elect to use oral contraceptives as a method of contraception.
- Oral contraceptives are highly effective for pregnancy prevention. Table 2 lists the typical accidental pregnancy rates for users of combination oral contraceptives and other methods of contraception. The efficacy of these contraceptive methods, except sterilization, the IUD, and the Norplant® System, depends upon the reliability with which they are used. Correct and consistent use of methods can result in lower failure rates.
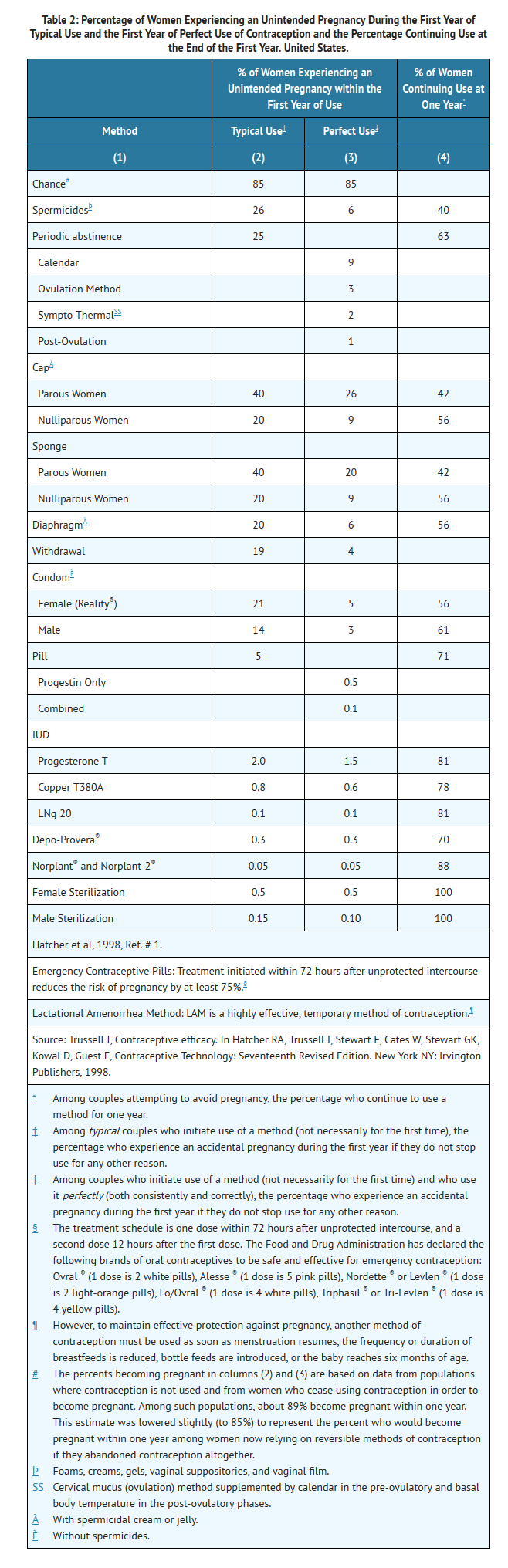
- MonoNessa® has not been studied for and is not indicated for use in emergency contraception.
- In clinical trials with MonoNessa®, 1,651 subjects completed 24,272 cycles and the overall use-efficacy (typical user efficacy) pregnancy rate was approximately 1 pregnancy per 100 women-years. This rate includes patients who did not take the drug correctly.
- Dosing information:
- Oral Contraception:
- To achieve maximum contraceptive effectiveness, MonoNessa® must be taken exactly as directed and at intervals not exceeding 24 hours. The possibility of ovulation and conception prior to initiation of medication should be considered. MonoNessa® is available in a blister card with a tablet dispenser which is preset for a Sunday Start. Day 1 Start is also provided.
- Sunday Start:
- When taking MonoNessa®, the first blue "active" tablet should be taken on the first Sunday after menstruation begins. If the period begins on Sunday, the first tablet should be taken that day. Take one active tablet daily for 21 days followed by one dark green inactive tablet daily for 7 days. After 28 tablets have been taken, a new course is started the next day (Sunday). For the first cycle of a Sunday Start regimen, another method of contraception should be used until after the first 7 consecutive days of administration.
- If the patient misses one (1) active tablet in Weeks 1, 2, or 3, the tablet should be taken as soon as she remembers. If the patient misses two (2) active tablets in Week 1 or Week 2, the patient should take two (2) tablets the day she remembers and two (2) tablets the next day; and then continue taking one (1) tablet a day until she finishes the pack. The patient should be instructed to use a back-up method of birth control such as a condom or spermicide if she has sex in the seven (7) days after missing pills. If the patient misses two (2) active tablets in the third week or misses three (3) or more active tablets in a row, the patient should continue taking one tablet every day until Sunday. On Sunday the patient should throw out the rest of the pack and start a new pack that same day. The patient should be instructed to use a back-up method of birth control if she has sex in the seven (7) days after missing pills.
- Complete instructions to facilitate patient counseling on proper pill usage may be found in the Detailed Patient Labeling ("How to Take the Pill" section).
- Day 1 Start:
- The dosage of MonoNessa® for the initial cycle of therapy is one blue "active" tablet administered daily from the 1st day through the 21st day of the menstrual cycle, counting the first day of menstrual flow as "Day 1" followed by one dark green inactive tablet daily for 7 days. Tablets are taken without interruption for 28 days. After 28 tablets have been taken, a new course is started the next day.
- If the patient misses one (1) active tablet in Weeks 1, 2, or 3, the tablet should be taken as soon as she remembers. If the patient misses two (2) active tablets in Week 1 or Week 2, the patient should take two (2) tablets the day she remembers and two (2) tablets the next day; and then continue taking one (1) tablet a day until she finishes the pack. The patient should be instructed to use a back-up method of birth control such as a condom or spermicide if she has sex in the seven (7) days after missing pills. If the patient misses two (2) active tablets in the third week or misses three (3) or more active tablets in a row, the patient should throw out the rest of the pack and start a new pack that same day. The patient should be instructed to use a back-up method of birth control if she has sex in the seven (7) days after missing pills.
- Complete instructions to facilitate patient counseling on proper pill usage may be found in the Detailed Patient Labeling ("How to Take the Pill" section).
- The use of MonoNessa® for contraception may be initiated 4 weeks postpartum in women who elect not to breastfeed. When the tablets are administered during the postpartum period, the increased risk of thromboembolic disease associated with the postpartum period must be considered. The possibility of ovulation and conception prior to initiation of medication should be considered.
- (See Discussion of Dose-Related Risk of Vascular Disease from Oral Contraceptives.)
Acne
- Dosing information:
- Ortho Tri-Cyclen(R), 1 tablet ORALLY daily at the same time; first week, white active tablets; second week, light blue active tablets; third week, blue active tablets; fourth week, green inactive tablets; repeat course monthly
NON-CONTRACEPTIVE HEALTH BENEFITS
- The following non-contraceptive health benefits related to the use of combination oral contraceptives are supported by epidemiological studies which largely utilized oral contraceptive formulations containing estrogen doses exceeding 0.035 mg of ethinyl estradiol or 0.05 mg mestranol.73–78
- Effects on menses:
- increased menstrual cycle regularity
- decreased blood loss and decreased incidence of iron deficiency anemia
- decreased incidence of dysmenorrhea
- Effects related to inhibition of ovulation:
- decreased incidence of functional ovarian cysts
- decreased incidence of ectopic pregnancies
- Other effects:
- decreased incidence of fibroadenomas and fibrocystic disease of the breast
- decreased incidence of acute pelvic inflammatory disease
- decreased incidence of endometrial cancer
- decreased incidence of ovarian cancer
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
- There is limited information regarding Off-Label Guideline-Supported Use of Norgestimate and Ethinyl estradiol in adult patients.
Non–Guideline-Supported Use
- There is limited information regarding Off-Label Non–Guideline-Supported Use of Norgestimate and Ethinyl estradiol in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
There is limited information regarding Norgestimate and Ethinyl estradiol FDA-Labeled Indications and Dosage (Pediatric) in the drug label.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
- There is limited information regarding Off-Label Guideline-Supported Use of Norgestimate and Ethinyl estradiol in pediatric patients.
Non–Guideline-Supported Use
- There is limited information regarding Off-Label Non–Guideline-Supported Use of Norgestimate and Ethinyl estradiol in pediatric patients.
Contraindications
- Oral contraceptives should not be used in women who currently have the following conditions:
- Thrombophlebitis or thromboembolic disorders
- A past history of deep vein thrombophlebitis or thromboembolic disorders
- Known thrombophilic conditions
- Cerebral vascular or coronary artery disease (current or past history)
- Valvular heart disease with complications
- Persistent blood pressure values of ≥ 160 mm Hg systolic or ≥ 100 mg Hg diastolic 102
- Diabetes with vascular involvement
- Headaches with focal neurological symptoms
- Major surgery with prolonged immobilization
- Known or suspected carcinoma of the breast or personal history of breast cancer
- Carcinoma of the endometrium or other known or suspected estrogen-dependent neoplasia
- Undiagnosed abnormal genital bleeding
- Cholestatic jaundice of pregnancy or jaundice with prior pill use
- Acute or chronic hepatocellular disease with abnormal liver function
- Hepatic adenomas or carcinomas
- Known or suspected pregnancy
- Hypersensitivity to any component of this product
Warnings
|
WARNINGS: CARDIOVASCULAR RISK ASSOCIATED WITH SMOKING
See full prescribing information for complete Boxed Warning.
Cardiovascular risk associated with smoking:
|
- Cigarette smoking increases the risk of serious cardiovascular events from combination oral contraceptive use. This risk increases with age, particularly in women over 35 years of age, and with the number of cigarettes smoked. For this reason, combination oral contraceptives, including MonoNessa®, should not be used by women who are over 35 years of age and smoke.
- The use of oral contraceptives is associated with increased risks of several serious conditions including myocardial infarction, thromboembolism, stroke, hepatic neoplasia, and gallbladder disease, although the risk of serious morbidity or mortality is very small in healthy women without underlying risk factors. The risk of morbidity and mortality increases significantly in the presence of other underlying risk factors such as hypertension, hyperlipidemias, obesity and diabetes.
- Practitioners prescribing oral contraceptives should be familiar with the following information relating to these risks.
- The information contained in this package insert is principally based on studies carried out in patients who used oral contraceptives with higher formulations of estrogens and progestogens than those in common use today. The effect of long-term use of the oral contraceptives with lower formulations of both estrogens and progestogens remains to be determined.
- Throughout this labeling, epidemiological studies reported are of two types: retrospective or case control studies and prospective or cohort studies. Case control studies provide a measure of the relative risk of a disease, namely, a ratio of the incidence of a disease among oral contraceptive users to that among nonusers. The relative risk does not provide information on the actual clinical occurrence of a disease. Cohort studies provide a measure of attributable risk, which is the difference in the incidence of disease between oral contraceptive users and nonusers. The attributable risk does provide information about the actual occurrence of a disease in the population (adapted from refs. 2 and 3 with the author's permission). For further information, the reader is referred to a text on epidemiological methods.
Thromboembolic Disorders and Other Vascular Problems
Myocardial Infarction
- An increased risk of myocardial infarction has been attributed to oral contraceptive use. This risk is primarily in smokers or women with other underlying risk factors for coronary artery disease such as hypertension, hypercholesterolemia, morbid obesity, and diabetes. The relative risk of heart attack for current oral contraceptive users has been estimated to be two to six.4–10 The risk is very low under the age of 30.
- Smoking in combination with oral contraceptive use has been shown to contribute substantially to the incidence of myocardial infarctions in women in their mid-thirties or older with smoking accounting for the majority of excess cases.11 Mortality rates associated with circulatory disease have been shown to increase substantially in smokers, especially in those 35 years of age and older, and in nonsmokers over the age of 40 among women who use oral contraceptives. (See Figure 1).
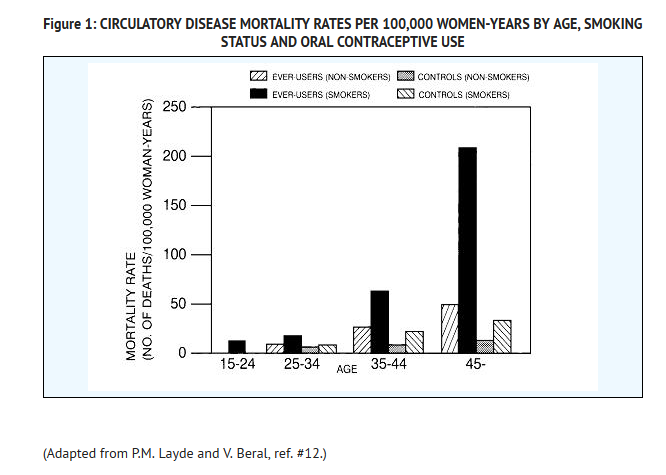
- Oral contraceptives may compound the effects of well-known risk factors, such as hypertension, diabetes, hyperlipidemias, age and obesity.13 In particular, some progestogens are known to decrease HDL cholesterol and cause glucose intolerance, while estrogens may create a state of hyperinsulinism.14–18 Oral contraceptives have been shown to increase blood pressure among users (see Section 9 in WARNINGS). Similar effects on risk factors have been associated with an increased risk of heart disease. Oral contraceptives must be used with caution in women with cardiovascular disease risk factors.
- Norgestimate has minimal androgenic activity, and there is some evidence that the risk of myocardial infarction associated with oral contraceptives is lower when the progestogen has minimal androgenic activity than when the activity is greater.97
Thromboembolism
- An increased risk of thromboembolic and thrombotic disease associated with the use of oral contraceptives is well established. Case control studies have found the relative risk of users compared to nonusers to be 3 for the first episode of superficial venous thrombosis, 4 to 11 for deep vein thrombosis or pulmonary embolism, and 1.5 to 6 for women with predisposing conditions for venous thromboembolic disease.2,3,19–24 Cohort studies have shown the relative risk to be somewhat lower, about 3 for new cases and about 4.5 for new cases requiring hospitalization.25 The risk of thromboembolic disease associated with oral contraceptives is not related to length of use and disappears after pill use is stopped.2
- A two- to four-fold increase in relative risk of post-operative thromboembolic complications has been reported with the use of oral contraceptives.9 The relative risk of venous thrombosis in women who have predisposing conditions is twice that of women without such medical conditions.26 If feasible, oral contraceptives should be discontinued at least four weeks prior to and for two weeks after elective surgery of a type associated with an increase in risk of thromboembolism and during and following prolonged immobilization. Since the immediate postpartum period is also associated with an increased risk of thromboembolism, oral contraceptives should be started no earlier than four weeks after delivery in women who elect not to breastfeed.
Cerebrovascular Diseases
- Oral contraceptives have been shown to increase both the relative and attributable risks of cerebrovascular events (thrombotic and hemorrhagic strokes), although, in general, the risk is greatest among older (>35 years), hypertensive women who also smoke. Hypertension was found to be a risk factor for both users and nonusers, for both types of strokes, and smoking interacted to increase the risk of stroke.27–29
- In a large study, the relative risk of thrombotic strokes has been shown to range from 3 for normotensive users to 14 for users with severe hypertension.30 The relative risk of hemorrhagic stroke is reported to be 1.2 for non-smokers who used oral contraceptives, 2.6 for smokers who did not use oral contraceptives, 7.6 for smokers who used oral contraceptives, 1.8 for normotensive users and 25.7 for users with severe hypertension.30 The attributable risk is also greater in older women.3
Dose-Related Risk of Vascular Disease From Oral Contraceptives
- A positive association has been observed between the amount of estrogen and progestogen in oral contraceptives and the risk of vascular disease.31–33 A decline in serum high density lipoproteins (HDL) has been reported with many progestational agents.14–16 A decline in serum high density lipoproteins has been associated with an increased incidence of ischemic heart disease. Because estrogens increase HDL cholesterol, the net effect of an oral contraceptive depends on a balance achieved between doses of estrogen and progestogen and the activity of the progestogen used in the contraceptives. The activity and amount of both hormones should be considered in the choice of an oral contraceptive.
- Minimizing exposure to estrogen and progestogen is in keeping with good principles of therapeutics. For any particular estrogen/progestogen combination, the dosage regimen prescribed should be one which contains the least amount of estrogen and progestogen that is compatible with a low failure rate and the needs of the individual patient. New acceptors of oral contraceptive agents should be started on preparations containing the lowest estrogen content which is judged appropriate for the individual patient.
Persistence of Risk of Vascular Disease
- There are two studies which have shown persistence of risk of vascular disease for ever-users of oral contraceptives. In a study in the United States, the risk of developing myocardial infarction after discontinuing oral contraceptives persists for at least 9 years for women 40–49 years who had used oral contraceptives for five or more years, but this increased risk was not demonstrated in other age groups.8 In another study in Great Britain, the risk of developing cerebrovascular disease persisted for at least 6 years after discontinuation of oral contraceptives, although excess risk was very small.34 However, both studies were performed with oral contraceptive formulations containing 50 micrograms or higher of estrogens.
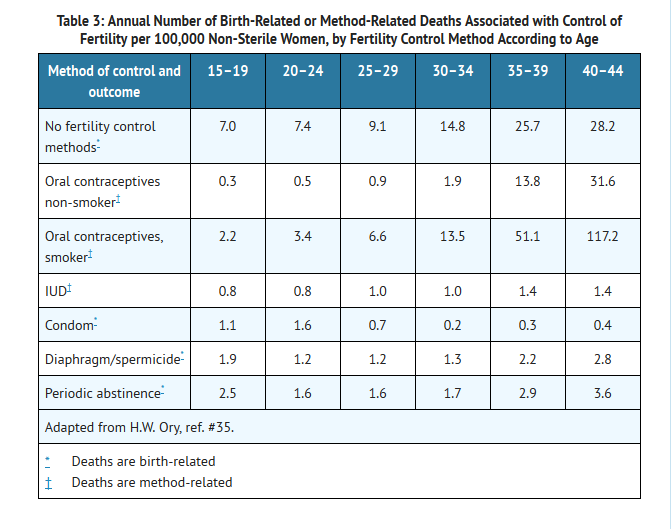
Estimates of Mortality From Contraceptive Use
- One study gathered data from a variety of sources which have estimated the mortality rate associated with different methods of contraception at different ages (Table 3). These estimates include the combined risk of death associated with contraceptive methods plus the risk attributable to pregnancy in the event of method failure. Each method of contraception has its specific benefits and risks. The study concluded that with the exception of oral contraceptive users 35 and older who smoke, and 40 and older who do not smoke, mortality associated with all methods of birth control is low and below that associated with childbirth. The observation of an increase in risk of mortality with age for oral contraceptive users is based on data gathered in the 1970's.35 Current clinical recommendation involves the use of lower estrogen dose formulations and a careful consideration of risk factors. In 1989, the Fertility and Maternal Health Drugs Advisory Committee was asked to review the use of oral contraceptives in women 40 years of age and over. The Committee concluded that although cardiovascular disease risks may be increased with oral contraceptive use after age 40 in healthy non-smoking women (even with the newer low-dose formulations), there are also greater potential health risks associated with pregnancy in older women and with the alternative surgical and medical procedures which may be necessary if such women do not have access to effective and acceptable means of contraception. The Committee recommended that the benefits of low-dose oral contraceptive use by healthy non-smoking women over 40 may outweigh the possible risks.
- Of course, older women, as all women, who take oral contraceptives, should take an oral contraceptive which contains the least amount of estrogen and progestogen that is compatible with a low failure rate and individual patient needs.
Carcinoma of the Reproductive Organs and Breasts
- Numerous epidemiological studies have been performed on the incidence of breast, endometrial, ovarian, and cervical cancer in women using oral contraceptives. The risk of having breast cancer diagnosed may be slightly increased among current and recent users of combination oral contraceptives (COCs). However, this excess risk appears to decrease over time after COC discontinuation and by 10 years after cessation the increased risk disappears. Some studies report an increased risk with duration of use while other studies do not and no consistent relationships have been found with dose or type of steroid. Some studies have found a small increase in risk for women who first use COCs before age 20. Most studies show a similar pattern of risk with COC use regardless of a woman's reproductive history or her family breast cancer history.
- Breast cancers diagnosed in current or previous oral contraceptive users tend to be less clinically advanced than in nonusers. Women who currently have or have had breast cancer should not use oral contraceptives because breast cancer is usually a hormonally-sensitive tumor.
- Some studies suggest that oral contraceptive use has been associated with an increase in the risk of cervical intraepithelial neoplasia in some populations of women.45–48 However, there continues to be controversy about the extent to which such findings may be due to differences in sexual behavior and other factors. In spite of many studies of the relationship between oral contraceptive use and breast and cervical cancers, a cause-and-effect relationship has not been established.
Hepatic Neoplasia
- Benign hepatic adenomas are associated with oral contraceptive use, although the incidence of benign tumors is rare in the United States. Indirect calculations have estimated the attributable risk to be in the range of 3.3 cases/100,000 for users, a risk that increases after four or more years of use especially with oral contraceptives of higher dose.49 Rupture of benign, hepatic adenomas may cause death through intra-abdominal hemorrhage.50,51
- Studies from Britain have shown an increased risk of developing hepatocellular carcinoma in long-term (>8 years) oral contraceptive users. However, these cancers are extremely rare in the U.S. and the attributable risk (the excess incidence) of liver cancers in oral contraceptive users approaches less than one per million users.
Ocular Lesions
- There have been clinical case reports of retinal thrombosis associated with the use of oral contraceptives. Oral contraceptives should be discontinued if there is unexplained partial or complete loss of vision; onset of proptosis or diplopia; papilledema; or retinal vascular lesions. Appropriate diagnostic and therapeutic measures should be undertaken immediately.
Oral Contraceptive Use Before or During Early Pregnancy
- Extensive epidemiological studies have revealed no increased risk of birth defects in women who have used oral contraceptives prior to pregnancy.56,57 The majority of recent studies also do not indicate a teratogenic effect, particularly in so far as cardiac anomalies and limb reduction defects are concerned55,56,58,59, when taken inadvertently during early pregnancy.
- The administration of oral contraceptives to induce withdrawal bleeding should not be used as a test for pregnancy. Oral contraceptives should not be used during pregnancy to treat threatened or habitual abortion.
- It is recommended that for any patient who has missed two consecutive periods, pregnancy should be ruled out. If the patient has not adhered to the prescribed schedule, the possibility of pregnancy should be considered at the time of the first missed period. Oral contraceptive use should be discontinued if pregnancy is confirmed.
Gallbladder Disease
- Earlier studies have reported an increased lifetime relative risk of gallbladder surgery in users of oral contraceptives and estrogens.60,61 More recent studies, however, have shown that the relative risk of developing gallbladder disease among oral contraceptive users may be minimal.62–64 The recent findings of minimal risk may be related to the use of oral contraceptive formulations containing lower hormonal doses of estrogens and progestogens.
Carbohydrate and Lipid Metabolic Effects
- Oral contraceptives have been shown to cause a decrease in glucose tolerance in a significant percentage of users.17 This effect has been shown to be directly related to estrogen dose.65 Progestogens increase insulin secretion and create insulin resistance, this effect varying with different progestational agents.17,66 However, in the non-diabetic woman, oral contraceptives appear to have no effect on fasting blood glucose.67 Because of these demonstrated effects, prediabetic and diabetic women in particular should be carefully monitored while taking oral contraceptives.
- A small proportion of women will have persistent hypertriglyceridemia while on the pill. As discussed earlier (see WARNINGS 1a and 1d), changes in serum triglycerides and lipoprotein levels have been reported in oral contraceptive users.
- In clinical studies with MonoNessa® there were no clinically significant changes in fasting blood glucose levels. No statistically significant changes in mean fasting blood glucose levels were observed over 24 cycles of use. Glucose tolerance tests showed minimal, clinically insignificant changes from baseline to cycles 3, 12, and 24.
Elevated Blood Pressure
- Women with significant hypertension should not be started on hormonal contraception.98 An increase in blood pressure has been reported in women taking oral contraceptives68 and this increase is more likely in older oral contraceptive users69 and with extended duration of use.61 Data from the Royal College of General Practitioners12 and subsequent randomized trials have shown that the incidence of hypertension increases with increasing progestational activity.
- Women with a history of hypertension or hypertension-related diseases, or renal disease70 should be encouraged to use another method of contraception. If these women elect to use oral contraceptives, they should be monitored closely and if a clinically significant persistent elevation of blood pressure (BP) occurs (≥ 160 mm Hg systolic or ≥ 100 mm Hg diastolic) and cannot be adequately controlled, oral contraceptives should be discontinued. In general, women who develop hypertension during hormonal contraceptive therapy should be switched to a non-hormonal contraceptive. If other contraceptive methods are not suitable, hormonal contraceptive therapy may continue combined with antihypertensive therapy. Regular monitoring of BP throughout hormonal contraceptive therapy is recommended.102 For most women, elevated blood pressure will return to normal after stopping oral contraceptives, and there is no difference in the occurrence of hypertension between former and never users.68–71 It should be noted that in two separate large clinical trials (N=633 and N=911), no statistically significant changes in mean blood pressure were observed with MonoNessa®.
Headache
- The onset or exacerbation of migraine or development of headache with a new pattern which is recurrent, persistent or severe requires discontinuation of oral contraceptives and evaluation of the cause.
Bleeding Irregularities
- Breakthrough bleeding and spotting are sometimes encountered in patients on oral contraceptives, especially during the first three months of use. Non-hormonal causes should be considered and adequate diagnostic measures taken to rule out malignancy or pregnancy in the event of breakthrough bleeding, as in the case of any abnormal vaginal bleeding. If pathology has been excluded, time or a change to another formulation may solve the problem. In the event of amenorrhea, pregnancy should be ruled out.
- Some women may encounter post-pill amenorrhea or oligomenorrhea, especially when such a condition was preexistent.
Ectopic Pregnancy
- Ectopic as well as intrauterine pregnancy may occur in contraceptive failures.
PRECAUTIONS
General
- Patients should be counseled that this product does not protect against HIV infection (AIDS) and other sexually transmitted diseases.
Physical Examination and Follow-up
- It is good medical practice for all women to have annual history and physical examinations, including women using oral contraceptives. The physical examination, however, may be deferred until after initiation of oral contraceptives if requested by the woman and judged appropriate by the clinician. The physical examination should include special reference to blood pressure, breasts, abdomen and pelvic organs, including cervical cytology, and relevant laboratory tests. In case of undiagnosed, persistent or recurrent abnormal vaginal bleeding, appropriate measures should be conducted to rule out malignancy. Women with a strong family history of breast cancer or who have breast nodules should be monitored with particular care.
Lipid Disorders
- Women who are being treated for hyperlipidemias should be followed closely if they elect to use oral contraceptives. Some progestogens may elevate LDL levels and may render the control of hyperlipidemias more difficult.
Liver Function
- If jaundice develops in any woman receiving such drugs, the medication should be discontinued. Steroid hormones may be poorly metabolized in patients with impaired liver function.
Fluid Retention
- Oral contraceptives may cause some degree of fluid retention. They should be prescribed with caution, and only with careful monitoring, in patients with conditions which might be aggravated by fluid retention.
Emotional Disorders
- Women with a history of depression should be carefully observed and the drug discontinued if depression recurs to a serious degree.
Contact Lenses
- Contact lens wearers who develop visual changes or changes in lens tolerance should be assessed by an ophthalmologist.
Interactions with Laboratory Tests
- Certain endocrine and liver function tests and blood components may be affected by oral contraceptives:
- Increased prothrombin and factors VII, VIII, IX, and X; decreased antithrombin 3; increased norepinephrine-induced platelet aggregability.
- Increased thyroid binding globulin (TBG) leading to increased circulating total thyroid hormone, as measured by protein-bound iodine (PBI), T4 by column or by radioimmunoassay. Free T3 resin uptake is decreased, reflecting the elevated TBG, free T4 concentration is unaltered.
- Other binding proteins may be elevated in serum.
- Sex hormone binding globulins are increased and result in elevated levels of total circulating sex steroids; however, free or biologically active levels either decrease or remain unchanged.
- Triglycerides may be increased and levels of various other lipids and lipoproteins may be affected.
- Glucose tolerance may be decreased.
- Serum folate levels may be depressed by oral contraceptive therapy. This may be of clinical significance if a woman becomes pregnant shortly after discontinuing oral contraceptives.
Adverse Reactions
Clinical Trials Experience
- An increased risk of the following serious adverse reactions has been associated with the use of oral contraceptives (see WARNINGS).
- Thrombophlebitis and venous thrombosis with or without embolism
- Arterial thromboembolism
- Pulmonary embolism
- Myocardial infarction
- Cerebral hemorrhage
- Cerebral thrombosis
- Hypertension
- Gallbladder disease
- Hepatic adenomas or benign liver tumors
- There is evidence of an association between the following conditions and the use of oral contraceptives:
- The following adverse reactions have been reported in patients receiving oral contraceptives and are believed to be drug-related:
- Nausea
- Vomiting
- Gastrointestinal symptoms (such as abdominal cramps and bloating)
- Breakthrough bleeding
- Spotting
- Change in menstrual flow
- Amenorrhea
- Temporary infertility after discontinuation of treatment
- Edema
- Melasma which may persist
- Breast changes: tenderness, enlargement, secretion
- Change in weight (increase or decrease)
- Change in cervical erosion and secretion
- Diminution in lactation when given immediately postpartum
- Cholestatic jaundice
- Migraine
- Allergic reaction, including rash, urticaria, angioedema
- Mental depression
- Reduced tolerance to carbohydrates
- Vaginal candidiasis
- Change in corneal curvature (steepening)
- Intolerance to contact lenses
- The following adverse reactions have been reported in users of oral contraceptives and a causal association has been neither confirmed nor refuted:
- Pre-menstrual syndrome
- Cataracts
- Changes in appetite
- Cystitis-like syndrome
- Headache
- Nervousness
- Dizziness
- Hirsutism
- Loss of scalp hair
- Erythema multiforme
- Erythema nodosum
- Hemorrhagic eruption
- Vaginitis
- Porphyria
- Impaired renal function
- Hemolytic uremic syndrome
- Acne
- Changes in libido
- Colitis
- Budd-Chiari Syndrome
- The following adverse reactions were also reported in clinical trials or during post-marketing experience: Infections and Infestations: vaginal infection, urinary tract infection; Psychiatric Disorders: mood altered, anxiety, insomnia; Gastrointestinal Disorders: flatulence, pancreatitis, diarrhea, constipation; Reproductive System and Breast Disorders: dysmenorrhea; ovarian cyst, vulvovaginal dryness; Neoplasms Benign, Malignant and Unspecified (Including Cysts and Polyps): benign breast neoplasm, fibro adenoma of breast, breast cyst; Nervous System Disorders: syncope, convulsion, paraesthesia; Eye Disorders: visual impairment, dry eye; Ear and Labyrinth Disorders: vertigo; Cardiac Disorders: tachycardia, palpitations; Vascular Disorders: hot flush; Respiratory, Thoracic and Mediastinal Disorders: dyspnea; Hepatobiliary Disorders: hepatitis; Skin and Subcutaneous Tissue Disorders: night sweats, hyperhidrosis, photosensitivity reaction, pruritus; Musculoskeletal, Connective Tissue, and Bone Disorders: muscle spasms, pain in extremity, myalgia, back pain; General Disorders and Administration Site Conditions: chest pain, asthenic conditions.
Postmarketing Experience
There is limited information regarding Norgestimate and Ethinyl estradiol Postmarketing Experience in the drug label.
Drug Interactions
There is limited information regarding Norgestimate and Ethinyl estradiol Drug Interactions in the drug label.
Use in Specific Populations
Pregnancy
Pregnancy Category (FDA): X Oral Contraceptive Use Before or During Early Pregnancy
- Extensive epidemiological studies have revealed no increased risk of birth defects in women who have used oral contraceptives prior to pregnancy.56,57 The majority of recent studies also do not indicate a teratogenic effect, particularly in so far as cardiac anomalies and limb reduction defects are concerned55,56,58,59, when taken inadvertently during early pregnancy.
- The administration of oral contraceptives to induce withdrawal bleeding should not be used as a test for pregnancy. Oral contraceptives should not be used during pregnancy to treat threatened or habitual abortion.
- It is recommended that for any patient who has missed two consecutive periods, pregnancy should be ruled out. If the patient has not adhered to the prescribed schedule, the possibility of pregnancy should be considered at the time of the first missed period. Oral contraceptive use should be discontinued if pregnancy is confirmed.
Pregnancy Category (AUS):
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Norgestimate and Ethinyl estradiol in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Norgestimate and Ethinyl estradiol during labor and delivery.
Nursing Mothers
- Small amounts of oral contraceptive steroids have been identified in the milk of nursing mothers and a few adverse effects on the child have been reported, including jaundice and breast enlargement. In addition, combination oral contraceptives given in the postpartum period may interfere with lactation by decreasing the quantity and quality of breast milk. If possible, the nursing mother should be advised not to use combination oral contraceptives but to use other forms of contraception until she has completely weaned her child.
Pediatric Use
- Safety and efficacy of MonoNessa® has been established in women of reproductive age. Safety and efficacy are expected to be the same for postpubertal adolescents under the age of 16 and for users 16 years and older. Use of this product before menarche is not indicated.
Geriatic Use
- This product has not been studied in women over 65 years of age and is not indicated in this population.
Gender
There is no FDA guidance on the use of Norgestimate and Ethinyl estradiol with respect to specific gender populations.
Race
There is no FDA guidance on the use of Norgestimate and Ethinyl estradiol with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Norgestimate and Ethinyl estradiol in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Norgestimate and Ethinyl estradiol in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Norgestimate and Ethinyl estradiol in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Norgestimate and Ethinyl estradiol in patients who are immunocompromised.
Administration and Monitoring
Administration
Oral Contraception
- To achieve maximum contraceptive effectiveness, MonoNessa® must be taken exactly as directed and at intervals not exceeding 24 hours. The possibility of ovulation and conception prior to initiation of medication should be considered. MonoNessa® is available in a blister card with a tablet dispenser which is preset for a Sunday Start. Day 1 Start is also provided.
Sunday Start
- When taking MonoNessa®, the first blue "active" tablet should be taken on the first Sunday after menstruation begins. If the period begins on Sunday, the first tablet should be taken that day. Take one active tablet daily for 21 days followed by one dark green inactive tablet daily for 7 days. After 28 tablets have been taken, a new course is started the next day (Sunday). For the first cycle of a Sunday Start regimen, another method of contraception should be used until after the first 7 consecutive days of administration.
- If the patient misses one (1) active tablet in Weeks 1, 2, or 3, the tablet should be taken as soon as she remembers. If the patient misses two (2) active tablets in Week 1 or Week 2, the patient should take two (2) tablets the day she remembers and two (2) tablets the next day; and then continue taking one (1) tablet a day until she finishes the pack. The patient should be instructed to use a back-up method of birth control such as a condom or spermicide if she has sex in the seven (7) days after missing pills. If the patient misses two (2) active tablets in the third week or misses three (3) or more active tablets in a row, the patient should continue taking one tablet every day until Sunday. On Sunday the patient should throw out the rest of the pack and start a new pack that same day. The patient should be instructed to use a back-up method of birth control if she has sex in the seven (7) days after missing pills.
- Complete instructions to facilitate patient counseling on proper pill usage may be found in the Detailed Patient Labeling ("How to Take the Pill" section).
Day 1 Start
- The dosage of MonoNessa® for the initial cycle of therapy is one blue "active" tablet administered daily from the 1st day through the 21st day of the menstrual cycle, counting the first day of menstrual flow as "Day 1" followed by one dark green inactive tablet daily for 7 days. Tablets are taken without interruption for 28 days. After 28 tablets have been taken, a new course is started the next day.
- If the patient misses one (1) active tablet in Weeks 1, 2, or 3, the tablet should be taken as soon as she remembers. If the patient misses two (2) active tablets in Week 1 or Week 2, the patient should take two (2) tablets the day she remembers and two (2) tablets the next day; and then continue taking one (1) tablet a day until she finishes the pack. The patient should be instructed to use a back-up method of birth control such as a condom or spermicide if she has sex in the seven (7) days after missing pills. If the patient misses two (2) active tablets in the third week or misses three (3) or more active tablets in a row, the patient should throw out the rest of the pack and start a new pack that same day. The patient should be instructed to use a back-up method of birth control if she has sex in the seven (7) days after missing pills.
- Complete instructions to facilitate patient counseling on proper pill usage may be found in the Detailed Patient Labeling ("How to Take the Pill" section).
- The use of MonoNessa® for contraception may be initiated 4 weeks postpartum in women who elect not to breastfeed. When the tablets are administered during the postpartum period, the increased risk of thromboembolic disease associated with the postpartum period must be considered. (See CONTRAINDICATIONS and WARNINGS concerning thromboembolic disease. See also PRECAUTIONS: Nursing Mothers.) The possibility of ovulation and conception prior to initiation of medication should be considered.
- (See Discussion of Dose-Related Risk of Vascular Disease from Oral Contraceptives.)
Monitoring
- Oral contraceptives have been shown to cause a decrease in glucose tolerance in a significant percentage of users. This effect has been shown to be directly related to estrogen dose.65 Progestogens increase insulin secretion and create insulin resistance, this effect varying with different progestational agents.17,66 However, in the non-diabetic woman, oral contraceptives appear to have no effect on fasting blood glucose.67 Because of these demonstrated effects, prediabetic and diabetic women in particular should be carefully monitored while taking oral contraceptives.
- If these women elect to use oral contraceptives, they should be monitored closely and if a clinically significant persistent elevation of blood pressure (BP) occurs (≥ 160 mm Hg systolic or ≥ 100 mm Hg diastolic) and cannot be adequately controlled, oral contraceptives should be discontinued. Regular monitoring of BP throughout hormonal contraceptive therapy is recommended.
- Women with a strong family history of breast cancer or who have breast nodules should be monitored with particular care.
- Oral contraceptives may cause some degree of fluid retention. They should be prescribed with caution, and only with careful monitoring, in patients with conditions which might be aggravated by fluid retention.
IV Compatibility
There is limited information regarding the compatibility of Norgestimate and Ethinyl estradiol and IV administrations.
Overdosage
- Serious ill effects have not been reported following acute ingestion of large doses of oral contraceptives by young children. Overdosage may cause nausea and withdrawal bleeding may occur in females.
Pharmacology
There is limited information regarding Norgestimate and Ethinyl estradiol Pharmacology in the drug label.
Mechanism of Action
Oral Contraception
- Combination oral contraceptives act by suppression of gonadotropins. Although the primary mechanism of this action is inhibition of ovulation, other alterations include changes in the cervical mucus (which increase the difficulty of sperm entry into the uterus) and the endometrium (which reduce the likelihood of implantation).
- Receptor binding studies, as well as studies in animals and humans, have shown that norgestimate and 17-deacetyl norgestimate, the major serum metabolite, combine high progestational activity with minimal intrinsic androgenicity.90–93 Norgestimate, in combination with ethinyl estradiol, does not counteract the estrogen-induced increases in sex hormone binding globulin (SHBG), resulting in lower serum testosterone.90,91,94
Structure
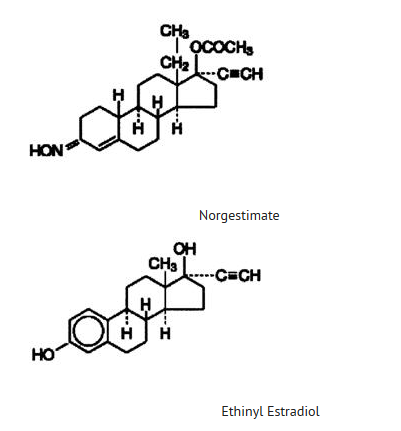
- The following product is a combination oral contraceptive containing the progestational compound norgestimate and the estrogenic compound ethinyl estradiol.
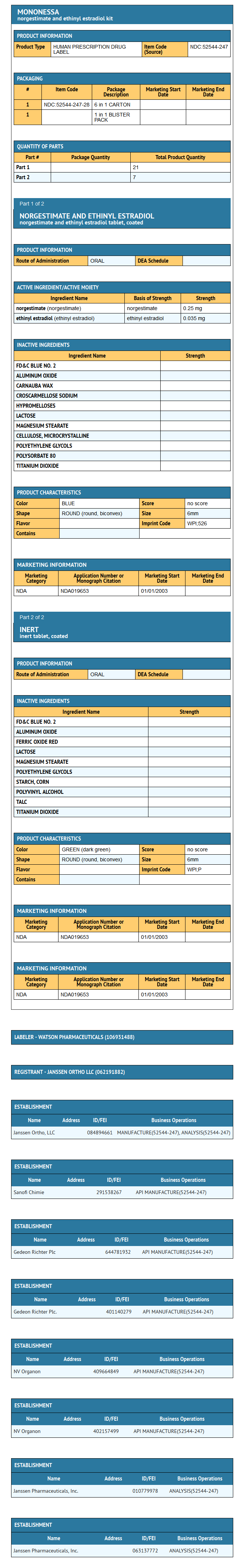
MonoNessa® Tablets
- Each blue tablet contains 0.250 mg of the progestational compound norgestimate (18,19-Dinor-17-pregn-4-en-20-yn-3-one,17-(acetyloxy)-13-ethyl-,oxime,(17α)-(+)-) and 0.035 mg of the estrogenic compound, ethinyl estradiol (19-nor-17α-pregna,1,3,5(10)-trien-20-yne-3,17-diol). Inactive ingredients include FD & C Blue No. 2 Aluminum Lake, carnauba wax, croscarmellose sodium, hypromellose, lactose, magnesium stearate, microcrystalline cellulose, polyethylene glycol, polysorbate 80, purified water and titanium dioxide.
- Each dark green tablet contains only inert ingredients, as follows: FD & C Blue No. 2 Aluminum Lake, ferric oxide, lactose, magnesium stearate, polyethylene glycol, pregelatinized corn starch, purified water, polyvinyl alcohol, talc and titanium dioxide.
Pharmacodynamics
There is limited information regarding Norgestimate and Ethinyl estradiol Pharmacodynamics in the drug label.
Pharmacokinetics
Absorption
- Norgestimate (NGM) and ethinyl estradiol (EE) are rapidly absorbed following oral administration. Norgestimate is rapidly and completely metabolized by first pass (intestinal and/or hepatic) mechanisms to norelgestromin (NGMN) and norgestrel (NG), which are the major active metabolites of norgestimate.
- Peak serum concentrations of NGMN and EE are generally reached by 2 hours after administration of MonoNessa®. Accumulation following multiple dosing of the 250 mcg NGM / 35 mcg dose is approximately 2-fold for NGMN and EE compared with single dose administration. The pharmacokinetics of NGMN is dose proportional following NGM doses of 180 mcg to 250 mcg. Steady-state concentration of EE is achieved by Day 7 of each dosing cycle. Steady-state concentrations of NGMN and NG are achieved by Day 21. Non-linear accumulation (approximately 8 fold) of norgestrel is observed as a result of high affinity binding to SHBG (sex hormone-binding globulin), which limits its biological activity.
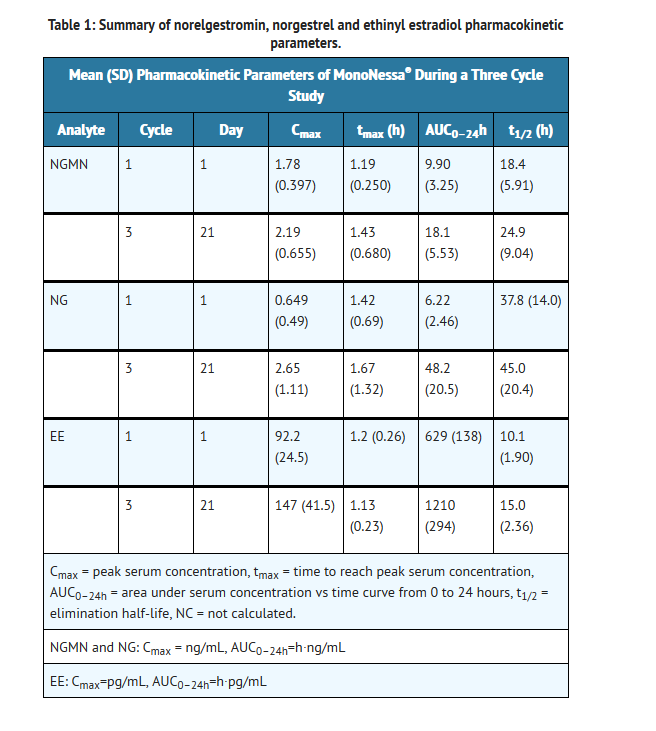
- The effect of food on the pharmacokinetics of MonoNessa® has not been studied.
Distribution
- Norelgestromin and norgestrel are highly bound (>97%) to serum proteins. Norelgestromin is bound to albumin and not to SHBG, while norgestrel is bound primarily to SHBG. Ethinyl estradiol is extensively bound (>97%) to serum albumin and induces an increase in the serum concentrations of SHBG.
Metabolism
- Norgestimate is extensively metabolized by first-pass mechanisms in the gastrointestinal tract and/or liver. Norgestimate's primary active metabolite is norelgestromin. Subsequent hepatic metabolism of norelgestromin occurs and metabolites include norgestrel, which is also active, and various hydroxylated and conjugated metabolites. Ethinyl estradiol is also metabolized to various hydroxylated products and their glucuronide and sulfate conjugates.
Excretion
- The metabolites of norelgestromin and ethinyl estradiol are eliminated by renal and fecal pathways. Following administration of 14C-norgestimate, 47% (45–49%) and 37% (16–49%) of the administered radioactivity was eliminated in the urine and feces, respectively. Unchanged norgestimate was not detected in the urine. In addition to 17-deacetyl norgestimate, a number of metabolites of norgestimate have been identified in human urine following administration of radiolabeled norgestimate. These include 18,19-Dinor-17-pregn-4-en-20-yn-3-one,17-hydroxy-13-ethyl,(17α)-(-);18,19-Dinor-5β-17-pregnan-20-yn,3α,17β-dihydroxy-13-ethyl,(17α), various hydroxylated metabolites and conjugates of these metabolites.
Special Populations
- The effects of body weight, body surface area or age on the pharmacokinetics of MonoNessa® have not been studied.
Hepatic Impairment
- The effects of hepatic impairment on the pharmacokinetics of MonoNessa® have not been studied. However, steroid hormones may be poorly metabolized in women with impaired liver function (see PRECAUTIONS).
Renal Impairment
- The effects of renal impairment on the pharmacokinetics of MonoNessa® have not been studied.
Drug-Drug Interactions
- No formal drug-drug interaction studies were conducted with MonoNessa®. Interactions between contraceptive steroids and other drugs have been reported in the literature (see PRECAUTIONS).
- bAlthough norelgestromin and its metabolites inhibit a variety of P450 enzymes in human liver microsomes, under the recommended dosing regimen, the in vivo concentrations of norelgestromin and its metabolites, even at the peak serum levels, are relatively low compared to the inhibitory constant (Ki).
Nonclinical Toxicology
There is limited information regarding Norgestimate and Ethinyl estradiol Nonclinical Toxicology in the drug label.
Clinical Studies
There is limited information regarding Norgestimate and Ethinyl estradiol Clinical Studies in the drug label.
How Supplied
- MonoNessa® is available in a blister card (NDC 52544-247-28) with a tablet dispenser (unfilled). The blister card contains 28 tablets as follows: 21 blue tablets containing 0.250 mg of the progestational compound, norgestimate, together with 0.035 mg of the estrogenic compound, ethinyl estradiol, which are unscored with "WPI" debossed on one side and "526" debossed on the opposite side, and 7 dark green tablets containing inert ingredients, which are unscored with "WPI" debossed on one side and "P:" debossed on the opposite side.
- 0.250/0.035 mg tablets - Blue, round, biconvex, coated tablet imprinted "WPI" on one side and "526" on the other side of the tablet.
- Each dark green reminder pill is a round, biconvex, coated tablet imprinted "WPI" on one side and "P" on the other side.
- Keep out of reach of children.
Storage
- Store at 25° C (77°F); excursions permitted to 15° – 30° C (59° – 86°F).
- Protect from light.
Images
Drug Images
{{#ask: Page Name::Norgestimate and Ethinyl estradiol |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel

{{#ask: Label Page::Norgestimate and Ethinyl estradiol |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
- Breakthrough bleeding, spotting, and amenorrhea are frequent reasons for patients discontinuing oral contraceptives. In breakthrough bleeding, as in all cases of irregular bleeding from the vagina, nonfunctional causes should be borne in mind. In undiagnosed persistent or recurrent abnormal bleeding from the vagina, adequate diagnostic measures are indicated to rule out pregnancy or malignancy. If pathology has been excluded, time or a change to another formulation may solve the problem. Changing to an oral contraceptive with a higher estrogen content, while potentially useful in minimizing menstrual irregularity, should be done only if necessary since this may increase the risk of thromboembolic disease.
- Use of oral contraceptives in the event of a missed menstrual period:
- If the patient has not adhered to the prescribed schedule, the possibility of pregnancy should be considered at the time of the first missed period and oral contraceptive use should be discontinued if pregnancy is confirmed.
- If the patient has adhered to the prescribed regimen and misses two consecutive periods, pregnancy should be ruled out.


Precautions with Alcohol
- Alcohol-Norgestimate and Ethinyl estradiol interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
Ortho-Cyclen, Ortho Tri-Cyclen, Ortho Tri-Cyclen Lo, Previfem, Tri-Previfem, Sprintec, Tri-Sprintec, MonoNessa.
Look-Alike Drug Names
There is limited information regarding Norgestimate and Ethinyl estradiol Look-Alike Drug Names in the drug label.
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.