Benzene
|
WikiDoc Resources for Benzene |
|
Articles |
|---|
|
Most recent articles on Benzene |
|
Media |
|
Evidence Based Medicine |
|
Clinical Trials |
|
Ongoing Trials on Benzene at Clinical Trials.gov Clinical Trials on Benzene at Google
|
|
Guidelines / Policies / Govt |
|
US National Guidelines Clearinghouse on Benzene
|
|
Books |
|
News |
|
Commentary |
|
Definitions |
|
Patient Resources / Community |
|
Directions to Hospitals Treating Benzene Risk calculators and risk factors for Benzene
|
|
Healthcare Provider Resources |
|
Causes & Risk Factors for Benzene |
|
Continuing Medical Education (CME) |
|
International |
|
|
|
Business |
|
Experimental / Informatics |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]
Overview
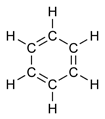
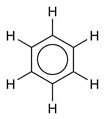
Benzene, or Benzol (see also Benzine) is an organic chemical compound with the formula C6H6. It is sometimes abbreviated Ph–H. Benzene is a colorless and flammable liquid with a sweet smell and a relatively high melting point. It is carcinogenic and its use as an additive in gasoline is now limited, but it is an important industrial solvent and precursor in the production of drugs, plastics, synthetic rubber, and dyes. Benzene is a natural constituent of crude oil, but it is usually synthesized from other compounds present in petroleum. Benzene is an aromatic hydrocarbon and the second [n]-annulene ([6]-annulene), a cyclic hydrocarbon with a continuous pi bond.
History
The word benzene derives historically from "gum benzoin", sometimes called "benjamin" (i.e., benzoin resin), an aromatic resin known to European pharmacists and perfumers since the fifteenth century as a product of southeast Asia. "Benzoin" is itself a corruption of the Arabic expression "luban jawi," or "frankincense of Java." An acidic material was derived from benzoin by sublimation, and named "flowers of benzoin," or benzoic acid. The hydrocarbon derived from benzoic acid thus acquired the name benzin, benzol, or benzene.[1]
Benzene has been the subject of many studies by scientists ranging from Michael Faraday to Linus Pauling. Faraday first isolated benzene in 1825 from the oily residue derived from the production of illuminating gas, giving it the name bicarburet of hydrogen.[2][3] In 1833, Eilhard Mitscherlich produced it via the distillation of benzoic acid (from gum benzoin) and lime. Mitscherlich gave the compound the name benzin.[4] In 1836 the French chemist Auguste Laurent named the substance "phène"; this is the root of the word phenol, which is hydroxylated benzene, and phenyl, which is the radical formed by abstraction of a hydrogen atom from benzene.
In 1845, Charles Mansfield, working under August Wilhelm von Hofmann, isolated benzene from coal tar. Four years later, Mansfield began the first industrial-scale production of benzene, based on the coal-tar method.
Gradually the sense developed among chemists that substances related to benzene formed a natural chemical family. In 1855 August Wilhelm Hofmann used the word "aromatic" to designate this family relationship, after a characteristic property of many of its members.
The empirical formula for benzene was long known, but its highly polyunsaturated structure was challenging to determine. Archibald Scott Couper in 1858 and Joseph Loschmidt in 1861 suggested possible structures that contained multiple double bonds or multiple rings, but the study of aromatic compounds was in its very early years, and too little evidence was then available to help chemists decide on any particular structure.
In 1865 the German chemist Friedrich August Kekulé published a paper in French (for he was then teaching in Francophone Belgium) suggesting that the structure contained a six-membered ring of carbon atoms with alternating single and double bonds. The next year he published a much longer paper in German on the same subject.[5][6] Kekulé used evidence that had accumulated in the intervening years—namely, that there always appeared to be only one isomer of any monoderivative of benzene, and that there always appeared to be exactly three isomers of every diderivative—to argue in support of his proposed structure. Kekulé's symmetrical ring could explain these curious facts.
The new understanding of benzene, and hence of all aromatic compounds, proved to be so important for both pure and applied chemistry that in 1890 the German Chemical Society organized an elaborate appreciation in Kekulé's honor, celebrating the twenty-fifth anniversary of his first benzene paper. Here Kekulé spoke of the creation of the theory. He said that he had discovered the ring shape of the benzene molecule after having a reverie or day-dream of a snake seizing its own tail (this is a common symbol in many ancient cultures known as the Ouroboros). This vision, he said, came to him after years of studying the nature of carbon-carbon bonds. This was 20 years after he had solved the problem of how carbon atoms could bond to up to four other atoms at the same time. It is curious that a similar humorous depiction of benzene had appeared in 1886 in the Berichte der Durstigen Chemischen Gesellschaft (Journal of the Thirsty Chemical Society), a parody of the Berichte der Deutschen Chemischen Gesellschaft, only the parody had monkeys seizing each other in a circle, rather than snakes as in Kekulé's anecdote.[7] Some historians have suggested that the parody was a lampoon of the snake anecdote, possibly already well-known through oral transmission even if it had not yet appeared in print.[1] Others have speculated that Kekulé's story in 1890 was a re-parody of the monkey spoof, and was a mere invention rather than a recollection of an event in his life.
Kekulé's 1890 speech[8] in which these anecdotes appeared has been translated into English.[9] If one takes the anecdote as the memory of a real event, circumstances mentioned in the story suggest that it must have happened early in 1862.[10]
The cyclic nature of benzene was finally confirmed by the eminent crystallographer Kathleen Lonsdale.[11][12]
Structure
Benzene represents a special problem in that, to account for all the bonds, there must be alternating double carbon bonds:
Using X-ray diffraction, researchers discovered that all of the carbon-carbon bonds in benzene are of the same length of 140 picometres (pm). The C–C bond lengths are greater than a double bond (135pm) but shorter than a single bond (147pm). This intermediate distance is explained by electron delocalization: the electrons for C–C bonding are distributed equally between each of the six carbon atoms. One representation is that the structure exists as a superposition of so-called resonance structures, rather than either form individually. This delocalisation of electrons is known as aromaticity, and gives benzene great stability. This enhanced stability is the fundamental property of aromatic molecules that differentiates them from molecules that are non-aromatic. To reflect the delocalised nature of the bonding, benzene is often depicted with a circle inside a hexagonal arrangement of carbon atoms:
As is common in organic chemistry, the carbon atoms in the diagram above have been left unlabeled. Benzene occurs sufficiently often as a component of organic molecules that there is a Unicode symbol with the code 232C to represent it:
Many fonts do not have this Unicode character, so many programs may not be able to display it correctly. A graphical representation of this symbol can be found at the following URL: http://www.fileformat.info/info/unicode/char/232c/index.htm
Substituted benzene derivatives
Many important chemicals are derived from benzene, wherein with one or more of the hydrogen atoms is replaced with another functional group. Examples of simple benzene derivatives are phenol, toluene, and aniline, abbreviated PhOH, PhMe, and PhNH2, respectively. Linking benzene rings gives biphenyl, C6H5–C6H5. Further loss of hydrogen gives "fused" aromatic hydrocarbons, such as naphthalene and anthracene. The limit of the fusion process is the hydrogen-free material graphite.
In heterocycles, carbon atoms in the benzene ring are replaced with other elements. The most important derivatives are the rings containing nitrogen. Replacing one CH with N gives the compound pyridine, C5H5N. Although benzene and pyridine are structurally related, benzene cannot be converted into pyridine. Replacement of a second CH bond with N gives, depending on the location of the second N, pyridazine, pyrimidine, and pyrazine.
Production
Trace amounts of benzene may result whenever carbon-rich materials undergo incomplete combustion. It is produced in volcanoes and forest fires, and is also a component of cigarette smoke.
Up until World War II, most benzene was produced as a byproduct of coke production (or "coke-oven light oil") in the steel industry. However, in the 1950s, increased demand for benzene, especially from the growing plastics industry, necessitated the production of benzene from petroleum. Today, most benzene comes from the petrochemical industry, with only a small fraction being produced from coal.
Three chemical processes contribute equally to industrial benzene production: catalytic reforming, toluene hydrodealkylation, and steam cracking.
Catalytic reforming
In catalytic reforming, a mixture of hydrocarbons with boiling points between 60–200 °C is blended with hydrogen gas and then exposed to a bifunctional platinum chloride or rhenium chloride catalyst at 500–525 °C and pressures ranging from 8–50 atm. Under these conditions, aliphatic hydrocarbons form rings and lose hydrogen to become aromatic hydrocarbons. The aromatic products of the reaction are then separated from the reaction mixture (or reformate) by extraction with any one of a number of solvents, including diethylene glycol or sulfolane, and benzene is then separated from the other aromatics by distillation. The extraction step of aromatics from the reformate is designed to produce aromatics with lowest non-aromatic components. So-called "BTX (Benzene-Toluene-Xylenes)" process consists of such extraction and distillation steps.
Similarly to this catalytic reforming, UOP and BP commercialized a method from LPG (mainly propane and butane) to aromatics.
Toluene hydrodealkylation
Toluene hydrodealkylation converts toluene to benzene. In this hydrogen-intensive process, toluene is mixed with hydrogen, then passed over a chromium, molybdenum, or platinum oxide catalyst at 500–600 °C and 40–60 atm pressure. Sometimes, higher temperatures are used instead of a catalyst (at the similar reaction condition). Under these conditions, toluene undergoes dealkylation according to the chemical equation:
This irreversible reaction is accompanied by an equilibrium side reaction that produces biphenyl (aka diphenyl) at higher temperature: 2 C6H6 ↔ H2 + C12H10
If the raw material stream contains much non-aromatic components (paraffins or naphthenes), those are likely decomposed to lower hydrocarbons such as methane, which increases the consumption of hydrogen.
A typical reaction yield exceeds 95%. Sometimes, xylenes and heavier aromatics are used in place of toluene, with similar efficiency.
This is often called "on-purpose" methodology to produce benzene, compared to conventional BTX (benzene-toluene-xylene) processes. The hydrodealkylation process is not economically feasible if the price gap between benzene and toluene is small (or the gap is smaller than about 15% of benzene price).
Toluene disproportionation
Where a chemical complex has similar demands for both benzene and xylene, then toluene disproportionation (TDP) may be an attractive alternative to the toluene hydrodealkylation. Broadly speaking 2 toluene molecules are reacted and the methyl groups rearranged from one toluene molecule to the other, yielding one benzene molecule and one xylene molecule.
Given that demand for para-xylene (p-xylene) substantially exceeds demand for other xylene isomers, a refinement of the TDP process called Selective TDP (STDP) may be used. In this process, the xylene stream exiting the TDP unit is approximately 90% paraxylene. In some current catalytic systems, even the benzene-to-xylenes ratio is decreased (more xylenes) when the demand of xylenes is higher.
Steam cracking
Steam cracking is the process for producing ethylene and other olefins from aliphatic hydrocarbons. Depending on the feedstock used to produce the olefins, steam cracking can produce a benzene-rich liquid byproduct called pyrolysis gasoline. Pyrolysis gasoline can be blended with other hydrocarbons as a gasoline additive, or distilled (in BTX process) to separate it into its components, including benzene.
Uses
Early uses
In the 19th and early-20th centuries, benzene was used as an after-shave lotion because of its pleasant smell. Prior to the 1920s, benzene was frequently used as an industrial solvent, especially for degreasing metal. As its toxicity became obvious, benzene was supplanted by other solvents, especially toluene (methyl benzene), which has similar physical properties but is not as carcinogenic.
In 1903, Ludwig Roselius popularized the use of benzene to decaffeinate coffee. This discovery led to the production of Sanka (the letters "ka" in the brand name stand for kaffein). This process was later discontinued.
As a petrol additive, benzene increases the octane rating and reduces knocking. Consequently, petrol often contained several percent benzene before the 1950s, when tetraethyl lead replaced it as the most widely-used antiknock additive. With the global phaseout of leaded petrol, benzene has made a comeback as a gasoline additive in some nations. In the United States, concern over its negative health effects and the possibility of benzene entering the groundwater have led to stringent regulation of petrol's benzene content, with limits typically around 1%. European petrol specifications now contain the same 1% limit on benzene content. The US EPA has new regulations that will lower the benzene content in gasoline to 0.62% in 2011.[13].
Current uses of benzene
Today benzene is mainly used as an intermediate to make other chemicals. Its most widely-produced derivatives include styrene, which is used to make polymers and plastics, phenol for resins and adhesives (via cumene), and cyclohexane, which is used in the manufacture of Nylon. Smaller amounts of benzene are used to make some types of rubbers, lubricants, dyes, detergents, drugs, explosives, napalm and pesticides.
In laboratory research, toluene is now often used as a substitute for benzene. The solvent-properties of the two are similar but toluene is less toxic and has a wider liquid range.
Benzene has been used as a basic research tool in a variety of experiments including analysis of a two-dimensional gas.
Used in watchmaking for the cleaning of hairsprings.
Reactions of benzene
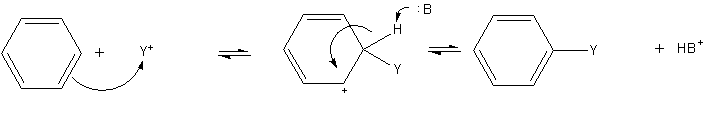
- Electrophilic aromatic substitution is a general method of derivatizing benzene. Benzene is sufficiently nucleophilic that it undergoes substitution by acylium ions or alkyl carbocations to give substituted derivatives.
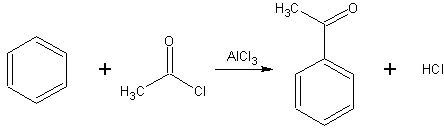
- The Friedel-Crafts acylation is a specific example of electrophilic aromatic substitution. The reaction involves the acylation of benzene (or many other aromatic rings) with an acyl chloride using a strong Lewis acid catalyst such as aluminium chloride or iron chloride which act as a halogen carrier.
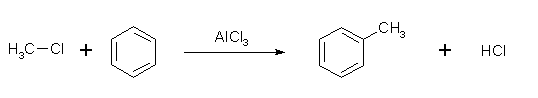
- Like the Friedel-Crafts acylation, the Friedel-Crafts alkylation involves the alkylation of benzene (and many other aromatic rings) using an alkyl halide in the presence of a strong Lewis acid catalyst.
- sulfonation.
- Nitration: Benzene undergoes nitration with nitronioum ions (NO2+) as the electrophile. Thus, warming benzene at 50-55 degrees Celsius, with a combination of concentrated sulphuric and nitric acid to produce the electrophile, gives nitrobenzene.
- Hydrogenation(Reduction): Benzene and derivatives convert to cyclohexane and derivatives when treated with hydrogen at 450K and 10atm of pressure with a finely divided nickel catalyst.
- Benzene is an excellent ligand in the organometallic chemistry of low-valent metals. Important examples include the sandwich and half-sandwich complexes respectively Cr(C6H6)2 and [RuCl2(C6H6)]2.
Health effects
Benzene exposure has serious health effects. Breathing high levels of benzene can result in death, while low levels can cause drowsiness, dizziness, rapid heart rate, headaches, tremors, confusion, and unconsciousness. Eating or drinking foods containing high levels of benzene can cause vomiting, irritation of the stomach, dizziness, sleepiness, convulsions, rapid heart rate, and death.
The major effects of benzene are chronic (long-term) exposure through the blood. Benzene damages the bone marrow and can cause a decrease in red blood cells, leading to anemia. It can also cause excessive bleeding and depress the immune system, increasing the chance of infection.
Some women who breathed high levels of benzene for many months had irregular menstrual periods and a decrease in the size of their ovaries. It is not known whether benzene exposure affects the developing fetus in pregnant women or fertility in men.
Animal studies have shown low birth weights, delayed bone formation, and bone marrow damage when pregnant animals breathed benzene.
The US Department of Health and Human Services (DHHS) classifies benzene as a human carcinogen. Long-term exposure to high levels of benzene in the air can cause leukemia, a potentially fatal cancer of the blood-forming organs. In particular, Acute myeloid leukemia or acute non-lymphocytic leukaemia (AML & ANLL) may be caused by benzene.
Several tests can determine exposure to benzene. There is a test for measuring benzene in the breath; this test must be done shortly after exposure. Benzene can also be measured in the blood; however, because benzene disappears rapidly from the blood, measurements are accurate only for recent exposures.
In the body, benzene is metabolized. Certain metabolites can be measured in the urine. However, this test must be done shortly after exposure and is not a reliable indicator of benzene exposure, since the same metabolites may be present in urine from other sources.
The United States Environmental Protection Agency has set the maximum permissible level of benzene in drinking water at 0.005 milligrams per liter (0.005 mg/L). The EPA requires that spills or accidental releases into the environment of 10 pounds (4.5 kg) or more of benzene be reported to the EPA.
The US Occupational Safety and Health Administration (OSHA) has set a permissible exposure limit of 0.5 part of benzene per million parts of air (.5 ppm) in the workplace during an 8-hour workday, 40-hour workweek. The short term exposure limit for airborne benzene is 5 ppm for 15 minutes.
In recent history there have been many examples of the harmful health effects of benzene and its derivatives. Toxic Oil Syndrome caused localised immune-suppression in Madrid in 1981 from people ingesting anilide-contaminated rapeseed oil. Chronic Fatigue Syndrome has also been highly correlated with people who eat "denatured" food that use solvents to remove fat or contain benzoic acid.
Workers in various industries that make or use benzene may be at risk for being exposed to high levels of this carcinogenic chemical. Industries that involve the use of benzene include the rubber industry, oil refineries, chemical plants, shoe manufacturers, and gasoline related industries. In 1987, OSHA estimated that about 237,000 workers in the United States were potentially exposed to benzene, and it is not known if this number has substantially changed since then.
Water and soil contamination are important pathways of concern for transmission of benzene contact. In the U.S. alone there are approximately 100,000 different sites which have benzene soil or groundwater contamination. In 2005, the water supply to the city of Harbin in China with a population of almost nine million people, was cut off because of a major benzene exposure. Benzene leaked into the Songhua River, which supplies drinking water to the city, after an explosion at a China National Petroleum Corporation (CNPC) factory in the city of Jilin on 13 November.
In March 2006, the official Food Standards Agency in Britain conducted a survey of 150 brands of soft drinks. It found that four contained benzene levels above World Health Organization limits. The affected batches were removed from sale. See benzene in soft drinks[14]
References
- ↑ 1.0 1.1 A. J. Rocke (1985). "Hypothesis and Experiment in the Early Development of Kekule's Benzene Theory". Annals of Science. 42: 355–81. doi:10.1080/00033798500200411.
- ↑ M. Faraday (1825). "On New Compounds of Carbon and Hydrogen, and on Certain Other Products Obtained during the Decomposition of Oil by Heat". Philosophical Transactions of the Royal Society of London. 115: 440–466.
- ↑ R. Kaiser (1968). "Bicarburet of Hydrogen. Reappraisal of the Discovery of Benzene in 1825 with the Analytical Methods of 1968". Angewandte Chemie International Edition in English. 7 (5): 345–350. doi:10.1002/anie.196803451.
- ↑ E. Mitscherlich (1834). "Ueber das Benzol und die Säuren der Oel- und Talgarten". Annalen der Pharmacie. 9 (1): 39–48. doi:10.1002/jlac.18340090103.
- ↑ F. A. Kekulé (1865). "Sur la constitution des substances aromatiques". Bulletin de la Societe Chimique de Paris. 3: 98–110.
- ↑ F. A. Kekulé (1866). "Untersuchungen uber aromatische Verbindungen". Liebigs Annalen der Chemie. 137: 129–36.
- ↑ Translated into English by D. Wilcox and F. Greenbaum, Journal of Chemical Education, 42 (1965), 266–67.
- ↑ F. A. Kekulé (1890). "Benzolfest: Rede". Berichte der Deutschen Chemischen Gesellschaft. 23: 1302–11.
- ↑ O. T. Benfey, "August Kekulé and the Birth of the Structural Theory of Organic Chemistry in 1858," Journal of Chemical Education, 35 (1958), 21–23
- ↑ Jean Gillis, "Auguste Kekulé et son oeuvre, realisee a Gand de 1858 a 1867," Memoires de l'Academie Royale de Belgique, 37:1 (1866), 1–40.
- ↑ K. Lonsdale (1929). "The Structure of the Benzene Ring in Hexamethylbenzene". Proceedings of the Royal Society. 123A: 494.
- ↑ K. Lonsdale (1931). "An X-Ray Analysis of the Structure of Hexachlorobenzene, Using the Fourier Method". Proceedings of the Royal Society. 133A: 536–553.
- ↑ Kolmetz, Gentry, Guidelines for BTX Revamps, AIChE 2007 Spring Conference
- ↑ "FDA: Too Much Benzene In Some Drinks", CBS News, May 19, 2006, retrieved July 11, 2006
External links
- ATSDR - Case Studies in Environmental Medicine: Benzene Toxicity
- Benzene
- Benzene Material Safety Data Sheet
- International Chemical Safety Card 0015
- MSAT Regulations and Remedies
- Australian National Pollutant Inventory - Benzene
- NIOSH Pocket Guide to Chemical Hazards
- IARC Monograph: "Benzene"
- Template:PubChemLink
- Computational Chemistry Wiki
- Couper and Carbon bonds
- Dept. of Health and Human Services: TR-289: Toxicology and Carcinogenesis Studies of Benzene
- Kekule, Couper and dreams of Benzene
- Loschmidt's Benzene structure
- Video Podcast (Sir John Cadogan giving a lecture on Benzene since Faraday, in 1991)
- Benzene 3D view and pdb-file
- National Institute for Occupational Safety and Health - Benzene Page
ar:بنزين (حلقة) bg:Бензен ca:Benzè cs:Benzen cy:Bensen da:Benzen de:Benzol el:Βενζόλιο eo:Benzeno fa:بنزن gl:Benceno ko:벤젠 hr:Benzen id:Benzena it:Benzene he:בנזן lv:Benzols lt:Benzenas hu:Benzol mk:Бензен ms:Benzena nl:Benzeen no:Benzen nn:Benzen simple:Benzene sk:Benzén sr:Бензен su:Bénzéna fi:Bentseeni sv:Bensen ta:பென்சீன் uk:Бензол ur:بنزین