Lorazepam (oral)
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Kiran Singh, M.D. [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Lorazepam (oral) is an antianxiety drug that is FDA approved for the treatment of anxiety disorders, status epilepticus, insomnia and as premedication for anesthetic procedure. Common adverse reactions include fatigue, drowsiness, amnesia, confusion, disorientation, depression, euphoria, ataxia, asthenia, tremor, and vertigo.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Indications
- Lorazepam is indicated for the management of anxiety disorders or for the short-term relief of the symptoms of anxiety or anxiety associated with depressive symptoms. Anxiety or tension associated with the stress of everyday life usually does not require treatment with an anxiolytic.
- The effectiveness of lorazepam in long-term use, that is, more than 4 months, has not been assessed by systematic clinical studies. The physician should periodically reassess the usefulness of the drug for the individual patient.
Dosage
- Lorazepam is administered orally. For optimal results, dose, frequency of administration, and duration of therapy should be individualized according to patient response. To facilitate this, 0.5 mg, 1 mg, and 2 mg tablets are available.
- The usual range is 2 to 6 mg/day given in divided doses, the largest dose being taken before bedtime, but the daily dosage may vary from 1 to 10 mg/day.
- For anxiety, most patients require an initial dose of 2 to 3 mg/day given b.i.d. or t.i.d.
- For insomnia due to anxiety or transient situational stress, a single daily dose of 2 to 4 mg may be given, usually at bedtime.
- For elderly or debilitated patients, an initial dosage of 1 to 2 mg/day in divided doses is recommended, to be adjusted as needed and tolerated.
- The dosage of lorazepam should be increased gradually when needed to help avoid adverse effects. When higher dosage is indicated, the evening dose should be increased before the daytime doses.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Lorazepam (oral) in adult patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Lorazepam (oral) in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
There is limited information regarding FDA-Labeled Use of Lorazepam (oral) in pediatric patients.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Lorazepam (oral) in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Lorazepam (oral) in pediatric patients.
Contraindications
- Lorazepam is contraindicated in patients with - - hypersensitivity to benzodiazepines or to any components of the formulation. - acute narrow-angle glaucoma.
Warnings
- Pre-existing depression may emerge or worsen during use of benzodiazepines including lorazepam. Lorazepam is not recommended for use in patients with a primary depressive disorder or psychosis.
- Use of benzodiazepines, including lorazepam, both used alone and in combination with other CNS depressants, may lead to potentially fatal respiratory depression.
- Use of benzodiazepines, including lorazepam, may lead to physical and psychological dependence.
- As with all patients on CNS-depressant drugs, patients receiving lorazepam should be warned not to operate dangerous machinery or motor vehicles and that their tolerance for alcohol and other CNS depressants will be diminished.
Physical And Psychological Dependence
- The use of benzodiazepines, including lorazepam, may lead to physical and psychological dependence. The risk of dependence increases with higher doses and longer term use and is further increased in patients with a history of alcoholism or drug abuse or in patients with significant personality disorders. The dependence potential is reduced when lorazepam is used at the appropriate dose for short-term treatment. Addiction-prone individuals (such as drug addicts or alcoholics) should be under careful surveillance when receiving lorazepam or other psychotropic agents.
- In general, benzodiazepines should be prescribed for short periods only (e.g. 2- 4 weeks). Extension of the treatment period should not take place without reevaluation of the need for continued therapy. Continuous long-term use of product is not recommended. Withdrawal symptoms (e.g. rebound insomnia) can appear following cessation of recommended doses after as little as one week of therapy. Abrupt discontinuation of product should be avoided and a gradual dosage-tapering schedule followed after extended therapy.
- Abrupt termination of treatment may be accompanied by withdrawal symptoms. Symptoms reported following discontinuation of benzodiazepines include headache, anxiety, tension, depression, insomnia, restlessness, confusion, irritability, sweating, rebound phenomena, dysphoria, dizziness, derealization, depersonalization, hyperacusis, numbness/ tingling of extremities, hypersensitivity to light, noise, and physical contact/perceptual changes, involuntary movements, nausea, vomiting, diarrhea, loss of appetite, hallucinations/delirium, convulsions/seizures, tremor, abdominal cramps, myalgia, agitation, palpitations, tachycardia, panic attacks, vertigo, hyperreflexia, short-term memory loss, and hyperthermia. Convulsions/seizures may be more common in patients with pre-existing seizure disorders or who are taking other drugs that lower the convulsive threshold such as antidepressants.
- There is evidence that tolerance develops to the sedative effects of benzodiazepines.
- Lorazepam may have abuse potential, especially in patients with a history of drug and/or alcohol
Adverse Reactions
Clinical Trials Experience
- Most adverse reactions to benzodiazepines, including CNS effects and respiratory depression, are dose dependent, with more severe effects occurring with high doses.
- In a sample of about 3500 patients treated for anxiety, the most frequent adverse reaction to lorazepam was sedation (15.9%), followed by dizziness (6.9%), weakness (4.2%), and unsteadiness (3.4%). The incidence of sedation and unsteadiness increased with age.
- Other adverse reactions to benzodiazepines, including lorazepam are fatigue, drowsiness, amnesia, memory impairment, confusion, disorientation, depression, unmasking of depression, disinhibition, euphoria, suicidal ideation/attempt, ataxia, asthenia, extrapyramidal symptoms, convulsions/seizures tremor, vertigo, eye-function/visual disturbance (including diplopia and blurred vision), dysarthria/slurred speech, change in libido, impotence, decreased orgasm; headache, coma; respiratory depression, apnea, worsening of sleep apnea, worsening of obstructive pulmonary disease; gastrointestinal symptoms including nausea, change in appetite, constipation, jaundice, increase in bilirubin, increase in liver transaminases, increase in alkaline phosphatase; hypersensitivity reactions, anaphylactic/oid reactions; dermatological symptoms, allergic skin reactions, alopecia; SIADH, hyponatremia; thrombocytopenia, agranulocytosis, pancytopenia; hypothermia; and autonomic manifestations.
- Paradoxical reactions, including anxiety, excitation, agitation, hostility, aggression, rage, sleep disturbances/insomnia, sexual arousal, and hallucinations may occur. Small decreases in blood pressure and hypotension may occur but are usually not clinically significant, probably being related to the relief of anxiety produced by lorazepam.
Postmarketing Experience
There is limited information regarding Lorazepam (oral) Postmarketing Experience in the drug label.
Drug Interactions
- The benzodiazepines, including lorazepam, produce increased CNS-depressant effects when administered with other CNS depressants such as alcohol, barbiturates, antipsychotics, sedative/hypnotics, anxiolytics, antidepressants, narcotic analgesics, sedative antihistamines, anticonvulsants, and anesthetics.
- Concomitant use of clozapine and lorazepam may produce marked sedation, excessive salivation, hypotension, ataxia, delirium, and respiratory arrest.
- Concurrent administration of lorazepam with valproate results in increased plasma concentrations and reduced clearance of lorazepam. Lorazepam dosage should be reduced to approximately 50% when coadministered with valproate.
- Concurrent administration of lorazepam with probenecid may result in a more rapid onset or prolonged effect of lorazepam due to increased half-life and decreased total clearance. Lorazepam dosage needs to be reduced by approximately 50% when coadministered with probenecid.
- The effects of probenecid and valproate on lorazepam may be due to inhibition of glucuronidation.
- Administration of theophylline or aminophylline may reduce the sedative effects of benzodiazepines, including lorazepam.
Use in Specific Populations
Pregnancy
- Reproductive studies in animals were performed in mice, rats, and two strains of rabbits. Occasional anomalies (reduction of tarsals, tibia, metatarsals, malrotated limbs, gastroschisis, malformed skull, and microphthalmia) were seen in drug-treated rabbits without relationship to dosage. Although all of these anomalies were not present in the concurrent control group, they have been reported to occur randomly in historical controls. At doses of 40 mg/kg and higher, there was evidence of fetal resorption and increased fetal loss in rabbits which was not seen at lower doses.
- The clinical significance of the above findings is not known. However, an increased risk of congenital malformations associated with the use of minor tranquilizers (chlordiazepoxide, diazepam, and meprobamate) during the first trimester of pregnancy has been suggested in several studies. Because the use of these drugs is rarely a matter of urgency, the use of lorazepam during this period should be avoided. The possibility that a woman of childbearing potential may be pregnant at the time of institution of therapy should be considered. Patients should be advised that if they become pregnant, they should communicate with their physician about the desirability of discontinuing the drug.
- In humans, blood levels obtained from umbilical cord blood indicate placental transfer of lorazepam and lorazepam glucuronide. Infants of mothers who ingested benzodiazepines for several weeks or more preceding delivery have been reported to have withdrawal symptoms during the postnatal period. Symptoms such as hypoactivity, hypotonia, hypothermia, respiratory depression, apnea, feeding problems, and impaired metabolic response to cold stress have been reported in neonates born of mothers who have received benzodiazepines during the late phase of pregnancy or at delivery.
Pregnancy Category (AUS):
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Lorazepam (oral) in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Lorazepam (oral) during labor and delivery.
Nursing Mothers
- Lorazepam has been detected in human breast milk; therefore, it should not be administered to breast-feeding women, unless the expected benefit to the woman outweighs the potential risk to the infant.
- Sedation and inability to suckle have occurred in neonates of lactating mothers taking benzodiazepines. Infants of lactating mothers should be observed for pharmacological effects (including sedation and irritability).
Pediatric Use
There is no FDA guidance on the use of Lorazepam (oral) in pediatric settings.
Geriatic Use
- Clinical studies of lorazepam generally were not adequate to determine whether subjects aged 65 and over respond differently than younger subjects; however, the incidence of sedation and unsteadiness was observed to increase with age.
- Age does not appear to have a significant effect on lorazepam kinetics.
- Clinical circumstances, some of which may be more common in the elderly, such as hepatic or renal impairment, should be considered. Greater sensitivity (e.g., sedation) of some older individuals cannot be ruled out. In general, dose selection for an elderly patient should be cautious, and lower doses may be sufficient in these patients
Gender
There is no FDA guidance on the use of Lorazepam (oral) with respect to specific gender populations.
Race
There is no FDA guidance on the use of Lorazepam (oral) with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Lorazepam (oral) in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Lorazepam (oral) in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Lorazepam (oral) in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Lorazepam (oral) in patients who are immunocompromised.
Administration and Monitoring
Administration
- Oral
Monitoring
There is limited information regarding Monitoring of Lorazepam (oral) in the drug label.
IV Compatibility
There is limited information regarding IV Compatibility of Lorazepam (oral) in the drug label.
Overdosage
- In postmarketing experience, overdose with lorazepam has occurred predominantly in combination with alcohol and/or other drugs. Therefore, in the management of overdosage, it should be borne in mind that multiple agents may have been taken.
Symptoms
- Overdosage of benzodiazepines is usually manifested by varying degrees of central nervous system depression ranging from drowsiness to coma. In mild cases, symptoms include drowsiness, mental confusion, paradoxical reactions, dysarthria and lethargy. In more serious cases, and especially when other drugs or alcohol were ingested, symptoms may include ataxia, hypotonia, hypotension, cardiovascular depression, respiratory depression, hypnotic state, coma, and death.
MANAGEMENT
- General supportive and symptomatic measures are recommended; vital signs must be monitored and the patient closely observed. When there is a risk of aspiration, induction of emesis is not recommended. Gastric lavage may be indicated if performed soon after ingestion or in symptomatic patients. Administration of activated charcoal may also limit drug absorption. Hypotension, though unlikely, usually may be controlled with norepinephrine bitartrate injection. Lorazepam is poorly dialyzable. Lorazepam glucuronide, the inactive metabolite, may be highly dialyzable.
- The benzodiazepine antagonist flumazenil may be used in hospitalized patients as an adjunct to, not as a substitute for, proper management of benzodiazepine overdose. The prescriber should be aware of a risk of seizure in association with flumazenil treatment, particularly in long-term benzodiazepine users and in cyclic antidepressant overdose.
Pharmacology
Mechanism of Action
There is limited information regarding Lorazepam (oral) Mechanism of Action in the drug label.
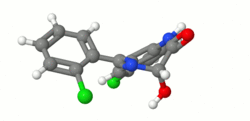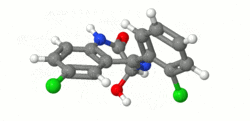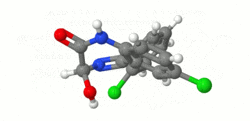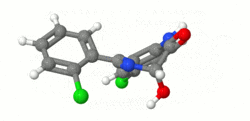
Structure
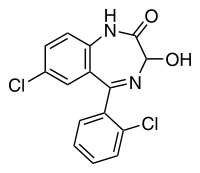
- Lorazepam, an antianxiety agent, has the chemical formula, 7-chloro-5-(o-chlorophenyl)-1,3-dihydro-3-hydroxy-2H-1,4-benzodiazepin-2-one:

- It is a nearly white powder almost insoluble in water. Each lorazepam tablet, to be taken orally, contains 0.5 mg, 1 mg or 2 mg of lorazepam. The inactive ingredients present are lactose, magnesium stearate, microcrystalline cellulose and polacrilin potassium.
Pharmacodynamics
There is limited information regarding Lorazepam (oral) Pharmacodynamics in the drug label.
Pharmacokinetics
There is limited information regarding Lorazepam (oral) Pharmacokinetics in the drug label.
Nonclinical Toxicology
- No evidence of carcinogenic potential emerged in rats during an 18-month study with lorazepam. No studies regarding mutagenesis have been performed.
Clinical Studies
There is limited information regarding Clinical Studies of Lorazepam (oral) in the drug label.
How Supplied
- Lorazepam Tablets USP are available in the following dosage strengths:
- 0.5 mg: white, scored, round flat faced beveled edge, debossed with 240 over 0.5 on one side and WATSON on the other side, supplied in bottles of 100, 500 and 1000.
- 1 mg: white, scored, round flat faced beveled edge, debossed with 241 over 1 on one side and WATSON on the other side, supplied in bottles of 100, 500 and 1000.
- 2 mg: white, scored, round flat faced beveled edge, debossed with 242 over 2 on one side and WATSON on the other side, supplied in bottles of 100, 500 and 1000.
Storage
- Store at 20°-25°C (68°-77°F).
- Dispense in a tight, light-resistant container as defined in the USP.
Images
Drug Images
{{#ask: Page Name::Lorazepam (oral) |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel


{{#ask: Label Page::Lorazepam (oral) |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
- To assure the safe and effective use of lorazepam, patients should be informed that, since benzodiazepines may produce psychological and physical dependence, it is advisable that they consult with their physician before either increasing the dose or abruptly discontinuing this drug.
Precautions with Alcohol
Alcohol-Lorazepam (oral) interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
- LORAZEPAM ®[4]
Look-Alike Drug Names
There is limited information regarding Lorazepam (oral) Look-Alike Drug Names in the drug label.
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
- ↑ Greenblatt DJ, Shader RI, Franke K, Maclaughlin DS, Harmatz JS, Allen MD, Werner A, Woo E (1991). "Pharmacokinetics and bioavailability of intravenous, intramuscular, and oral lorazepam in humans". Journal of Pharmaceutical Sciences. 68 (1): 57–63. doi:10.1002/jps.2600680119. PMID 31453.
- ↑ Greenblatt DJ, von Moltke LL, Ehrenberg BL, Harmatz JS, Corbett KE, Wallace DW, Shader RI (2000). "Kinetics and dynamics of lorazepam during and after continuous intravenous infusion". Critical Care Medicine. 28 (8): 2750–2757. doi:10.1097/00003246-200008000-00011. PMID 10966246.
- ↑ Papini O, da Cunha SP, da Silva Mathes Ado C, Bertucci C, Moisés EC, de Barros Duarte L, de Carvalho Cavalli R, Lanchote VL (2006). "Kinetic disposition of lorazepam with a focus on the glucuronidation capacity, transplacental transfer in parturients and racemization in biological samples". Journal of Pharmaceutical and Biomedical Analysis. 40 (2): 397–403. doi:10.1016/j.jpba.2005.07.021. PMID 16143486.
- ↑ "lorazepam tablet".