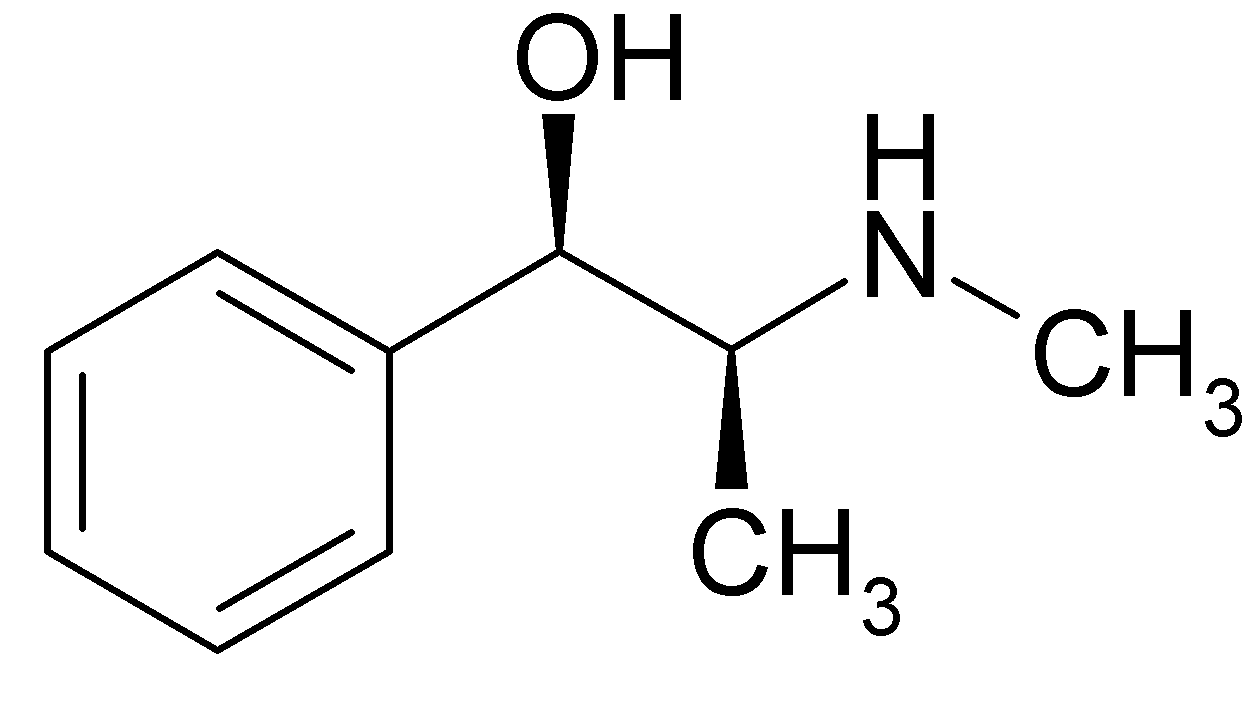
Ephedrine
 | |
 | |
| Clinical data | |
|---|---|
| Pregnancy category | |
| Routes of administration | oral, IV, IM, SC |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | 85% |
| Metabolism | minimal hepatic |
| Elimination half-life | 3–6 hours |
| Excretion | 22-99% renal |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| E number | {{#property:P628}} |
| ECHA InfoCard | {{#property:P2566}}Lua error in Module:EditAtWikidata at line 36: attempt to index field 'wikibase' (a nil value). |
| Chemical and physical data | |
| Formula | C10H15NO |
| Molar mass | 165.23 |
|
WikiDoc Resources for Ephedrine |
|
Articles |
|---|
|
Most recent articles on Ephedrine |
|
Media |
|
Evidence Based Medicine |
|
Clinical Trials |
|
Ongoing Trials on Ephedrine at Clinical Trials.gov Clinical Trials on Ephedrine at Google
|
|
Guidelines / Policies / Govt |
|
US National Guidelines Clearinghouse on Ephedrine
|
|
Books |
|
News |
|
Commentary |
|
Definitions |
|
Patient Resources / Community |
|
Patient resources on Ephedrine Discussion groups on Ephedrine Directions to Hospitals Treating Ephedrine Risk calculators and risk factors for Ephedrine
|
|
Healthcare Provider Resources |
|
Causes & Risk Factors for Ephedrine |
|
Continuing Medical Education (CME) |
|
International |
|
|
|
Business |
|
Experimental / Informatics |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]
Overview
Ephedrine (EPH) is a sympathomimetic amine similar in structure to the synthetic derivatives amphetamine and methamphetamine. Ephedrine is commonly used as a stimulant, appetite suppressant, concentration aid, decongestant and to treat hypotension associated with regional anaesthesia. Chemically, it is an alkaloid derived from various plants in the genus Ephedra (family Ephedraceae). It is most usually marketed in the hydrochloride and sulfate forms.
In traditional Chinese medicines, the herb ma huang (Ephedra sinica) contains ephedrine and pseudoephedrine as its principal active constituents. The same is true of other herbal products containing extracts from Ephedra species. Nagayoshi Nagai was the first one to isolate ephedrine from Ephedra vulgaris in 1885. The substance called soma mentioned in old Hindu books such as the Rig Veda, may have been ephedra extract.
The production of ephedrine in China has become a multi-million dollar export industry. Companies producing for export extract US$13 million worth of ephedrine from 30,000 tons of ephedra annually, 10 times the amount that is used in traditional Chinese medicine.[1]
Chemistry
Ephedrine exhibits optical isomerism and has two chiral centres. By convention the enantiomers with opposite stereochemistry around the chiral centres are designated ephedrine, while pseudoephedrine has same stereochemistry around the chiral carbons. That is, (1R,2R)- and (1S,2S)-enantiomers are designated pseudoephedrine; while (1R,2S)- and (1S,2R)-enantiomers are designated ephedrine.
The isomer which is marketed is (-)-(1R,2S)-ephedrine.[2]
As with other phenylethylamines, it is also somewhat chemically similar to methamphetamine, although the amphetamines are more potent and have additional biological effects.
Ephedrine may also be referred to as: (αR)-α-[(1S)-1-(methylamino)ethyl]benzenemethanol, α-[1-(methylamino)ethyl]benzyl alcohol, or L-erythro-2-(methylamino)-1-phenylpropan-1-ol. Ephedrine hydrochloride has a melting point of 187-188°C.[3]
Mode of action
Ephedrine is a sympathomimetic amine - that is, its principal mechanism of action relies on its direct and indirect actions on the adrenergic receptor system, which is part of the sympathetic nervous system or SNS. Central nervous system or CNS involvement is present, but the predominant clinical effects are caused by involvement with the sympathetic segment of the peripheral nervous system due to the fact that while ephedrine does cross the blood-brain barrier, it doesn't do this very efficiently (efficient crossers with similar modes of action would include amphetamine and methamphetamine).
Ephedrine increases post-synaptic noradrenergic receptor activity by (weakly) directly activating post-synaptic α-receptors and β-receptors, but the bulk of its effect comes from the pre-synaptic neuron being unable to distinguish between real adrenaline or noradrenaline from ephedrine. The ephedrine, mixed with noradrenaline, is transported through the noradrenaline reuptake complex and packaged (along with real noradrenaline) into vesicles that reside at the terminal button of a nerve cell.
As an alkaloid, having some small amount of ephedrine within a noradrenaline vesicle reduces the overall pH of the vesicle. This has the effect of increasing likelihood that the affected vesicle will be released during any subsequent action potential the nerve cell experiences. The nerve cells in question generally fire at some regular baseline rate; the effect of adding ephedrine is to increase the number of vesicles released during each action potential and possibly to extend the time during which noradrenaline has an opportunity to have an effect on the post-synaptic neuron by virtue of the fact that the reuptake complex has to process both noradrenaline AND ephedrine, presumably a longer process.
Ephedrine's mechanism of action on neurotransmission in the brain is wide. Its action as an agonist at most major noradrenaline receptors and its ability to increase the release of both dopamine and to a lesser extent, serotonin by the same mechanism as explained above for norepinephrine, is presumed to have a major role in its mechanism of action.
Because of ephedrine's ability to potentiate dopamine neurotransmission it is thought to have addictive properties by some researchers. The ability to potentiate serotonin and noradrenergic activity is clinically relevant, but is not thought to contribute to the potential for abuse.
While ephedrine's role in the serotonin system is less understood there is preliminary documentation of clinically significant agonism at excitory serotonin receptors, perhaps as a downstream response to the large release of norepinephrine in the nucleus accumbens (commonly referred to as the "pleasure center" of the brain). In mice, stereotypical behaviour was both easily induced by administration of ephedrine and it's primary alkaloids and reversed when serotonin antagonists were administered.
Clinical use

Indications
Ephedrine was once widely used as a topical decongestant and as a bronchodilator in the treatment for asthma. It continues to be used for these indications, although its popularity is waning due to the availability of more effective agents for these indications which exhibit fewer adverse effects.[4] The role in nasal congestion has largely been replaced by more potent α-adrenergic receptor agonists (e.g. oxymetazoline). Similarly the role of ephedrine in asthma has been almost entirely replaced by β2-adrenergic receptor agonists (e.g. salbutamol). Ephedrine continues to be used intravenously in the reversal of hypotension from spinal/epidural anaesthesia.[4] It is also used in other hypotensive states, including overdose with ganglionic blocking agents, antiadrenergic agents, or other medications that lower blood pressure.[5] It can be used in narcolepsy and nocturnal enuresis.
In traditional Chinese medicine, ephedrine has been used in the treatment of asthma and bronchitis for centuries.[6]
An ECA stack is a component found in thermogenic weight loss pills, composed of ephedrine, caffeine and aspirin (many supplement manufacturers include salicin instead of aspirin) working to speed up the metabolism and thus cause food energy to burn faster. The ECA stack is a popular supplement taken by body builders before workouts due to the increased amount of energy and alertness.
For many years, the US Coast Guard recommended ephedrine together with an equal 25 mg dose of promethazine to its sailors to combat seasickness. Promethazine manages nausea and ephedrine fights the ensuing drowsiness. Commonly referred to as the Coast Guard cocktail, ephedrine may still be available for prescription for this purpose.
Adverse effects
Adverse drug reactions (ADRs) are more common with systemic administration (e.g. injection or oral administration) compared to topical administration (e.g. nasal instillations). ADRs associated with ephedrine therapy include:[4]
- Cardiovascular: tachycardia, cardiac arrhythmias, Angina pectoris, vasoconstriction with hypertension
- Dermatological: flushing, sweating, acne vulgaris
- Gastrointestinal: nausea, appetite loss
- Genitourinary: increased urine output due to increased blood flow (difficulty urinating is not uncommon, as alpha-agonists such as ephedrine constrict the internal urethral sphincter, mimicking the effects of sympathetic nervous system stimulation)
- Nervous system:restlessness, confusion, insomnia, mild euphoria, mania/hallucinations (rare except in previously existing psychiatric conditions), delusions, formication (may be possible, but lacks documented evidence) paranoia, hostility, panic, agitation
- Respiratory: dyspnea, pulmonary edema
- Miscellaneous: dizziness, headache, tremor, hyperglycemic reactions
The approved maximum daily dosage of ephedrine for use as a bronchodilator is 150mg, as specified on the packaging of the bronchodilator and expectorant combination, Bronkaid, made by Bayer pharmaceuticals.
Overdose can lead to death, although the approved dose is not likely to cause severe reactions when used as directed.
Ephedrine can also lead to damage of the brain receptors over a period of high usage; this is because of its constant action on the neurochemicals. It also leads to high increase in blood pressure which over time can lead to damage in the blood vessels.
Contraindications
Ephedrine should not be used in conjunction with certain antidepressants, namely SNRIs (Selective norepinephrine re-uptake inhibitors), as this increases the risk of the above symptoms due to excessive serum levels of norepinephrine.
Wellbutrin is an example of an antidepressant with an amphetamine-like structure similar to ephedrine. It has a similar action but also releases serotonin from presynaptic clefts. It should not be used with ephedrine as it may increase the likelihood of the above side effects.
Ephedrine should be used with caution in patients with inadequate fluid replacement, impaired adrenal function, hypoxia, hypercapnia, acidosis, hypertension, hyperthyroidism, prostatic hypertrophy, diabetes mellitus, cardiovascular disease, during delivery if maternal BP > 130/80 mmHg, and lactation.[7]
Contraindications for the use of ephedrine include: closed angle glaucoma, phaeochromocytoma, asymmetric septal hypertrophy (idiopathic hypertrophic subaortic stenosis), concomitant or recent (previous 14 days) monoamine oxidase inhibitor (MAOI) therapy, general anaesthesia with halogenated hydrocarbons (particularly cyclopropane or halothane), tachyarrhythmias or ventricular fibrillation, hypersensitivity to ephedrine or other stimulants. Ephedrine should NOT be used at any time during pregnancy unless specifically indicated by a qualified physician and ONLY when other options are unavailable.[7]
Recreational and illicit use

Anecdotal reports have suggested that ephedrine helps studying, thinking, or concentrating to a greater extent than caffeine. Some students and some white-collar workers have used ephedrine (or Ephedra-containing herbal supplements) for this purpose, as well as some professional athletes and weightlifters. It is common for many athletes to use stimulants while exercising. Such use of ephedrine has been associated with stimulant dependence, as well as deaths from heatstroke in athletes and circulatory problems such as aortic aneurysm in weightlifters, though these side effects are rare.
As a phenylethylamine, ephedrine has a similar chemical structure to amphetamines. Ephedrine can be used in the synthesis of methamphetamine by chemical reduction; this has made ephedrine a highly sought-after chemical precursor in the illicit manufacture of methamphetamine. The most popular method for reducing ephedrine to methamphetamine is similar to the Birch reduction, in that it uses anhydrous ammonia and lithium metal in the reaction. The second most popular method uses red phosphorus, iodine, and ephedrine in the reaction.
Through oxidation, ephedrine can be easily synthesized into methcathinone. Ephedrine is listed as a Table I precursor under the United Nations Convention Against Illicit Traffic in Narcotic Drugs and Psychotropic Substances.[8]
Ephedrine has been reported to cause both physical and psychological dependence after excessive long-term use. This is particularly true with oral forms of ephedrine, since parenteral administration is unlikely to occur over long periods.[7]
Neurotoxicity of Ephedrine
As a sympathomimetic agent similar in structure and activity to amphetamines, there has been a dispute over whether ephedrine produces any neurodegenerative effects. It has not been shown clinically that certain amphetamines (namely (d)-amphetamine and (d)-methamphetamine) can cause varying levels of long-term dopamine depletion in dopamine-rich brain and nervous centers such as the putamen and the basal ganglia.
Several studies have recently compared the quantities of such neurotransmitters as serotonin, dopamine, glutamate, and epinephrine after concurrent administration of ephedrine and various amphetamine-like agents. The results showed that ephedrine has no neurotoxic effect nor has amphetamine counterparts.
Ephedrine increases serum dopamine levels minimally in comparison with an equivalent dose of dextroamphetamine (Dexedrine®). Dextromethamphetamine (Desoxyn®) raises dopamine levels dramatically (more than two times that of an equivalent dose of dextroamphetamine). This supports the general consensus that ephedrine has more of a peripheral action on the sympathetic nervous system, whereas amphetamines appear to cross the blood brain barrier more freely and tend to have a stronger central action. The fact that dopamine is believed to play a major role in the addiction response has been used in recent years as justification for controlling the distribution of dextroamphetamine and dextromethamphetamine, along with various other amphetamines.[9]
Legality in USA
Ephedrine itself has never been illegal in the United States. In 1997, the FDA proposed a regulation on ephedra (the herb from which ephedrine is obtained), which limited an ephedra dose to 8 mg (of active ephedrine) with no more than 24 mg per day.[2] This proposed rule was withdrawn in part in 2000 because of "concerns regarding the agency's basis for proposing a certain dietary ingredient level and a duration of use limit for these products."[3] In 2004, the FDA created a ban on ephedrine alkaloids that are marketed for reasons other than asthma, colds, allergies, other disease, or traditional Asian use.[4] On April 14, 2005, the U.S. District Court for the District of Utah ruled that the FDA did not have proper evidence that low dosages of ephedrine alkaloids are actually unsafe,[5] but on August 17, 2006, the U.S. Court of Appeals for the Tenth Circuit in Denver upheld the FDA's final rule declaring all dietary supplements containing ephedrine alkaloids adulterated, and therefore illegal for marketing in the United States.[6] Ephedrine is, however, still legal in many applications outside of dietary supplements. However, purchasing is currently limited and monitored, with specifics varying from state to state.
The House passed the Combat Methamphetamine Epidemic Act of 2005 as an amendment to the renewal of the USA PATRIOT Act. Signed into law by president George W. Bush on March 6, 2006, the act amended the US Code (21 USC 830) concerning the sale of ephedrine-containing products. The federal statute included the following requirements for merchants who sell these products:
- A retrievable record of all purchases identifying the name and address of each party to be kept for two years.
- Required verification of proof of identity of all purchasers
- Required protection and disclosure methods in the collection of personal information
- Reports to the Attorney General of any suspicious payments or disappearances of the regulated products
- Non-liquid dose form of regulated product may only be sold in unit dose blister packs
- Regulated products are to be sold behind the counter or in a locked cabinet in such a way as to restrict access
- Daily sales of regulated products not to exceed 3.6 grams without regard to the number of transactions
- Monthly sales not to exceed 9 grams of pseudoephedrine base in regulated products
The law gives similar regulations to mail-order purchases, except the monthly sales limit is only 7.5 grams.
References
- ↑ Long, Professor. http://www.chinadialogue.net/article/show/single/en/692-Chinese-medicine-s-great-waste-of-resources
- ↑ Edited by Reynolds JEF, ed. (1989). Martindale: The complete drug reference (29th edition ed.). London: Pharmaceutical Press. ISBN 0-85369-210-6.
- ↑ Budavari S, editor. The Merck Index: An encyclopedia of chemicals, drugs, and biologicals, 12th edition. Whitehouse Station: Merck
- ↑ 4.0 4.1 4.2 Joint Formulary Committee. British National Formulary, 47th edition. London: British Medical Association and Royal Pharmaceutical Society of Great Britain; 2004. ISBN 0853695873
- ↑ Bicopoulos D, editor. AusDI: Drug information for the healthcare professional, 2nd edition. Castle Hill: Pharmaceutical Care Information Services; 2002.
- ↑ Ford MD, Delaney KA, Ling LJ, Erickson T, editors. Clinical Toxicology. Philadelphia: WB Saunders; 2001. ISBN 0-7216-5485-1 Research Laboratories; 1996. ISBN 0-911910-12-3
- ↑ 7.0 7.1 7.2 Mayne Pharma. Ephedrine sulfate injection DBL (Approved Product Information). Melbourne: Mayne Pharma; 2004
- ↑ http://www.incb.org/pdf/e/list/red.pdf
- ↑ Txsci.oxfordjournals
See also
External links
- Pages with script errors
- Pages with reference errors
- CS1 maint: Extra text: editors list
- CS1 maint: Extra text
- E number from Wikidata
- ECHA InfoCard ID from Wikidata
- Chemical articles with unknown parameter in Infobox drug
- Articles without EBI source
- Chemical pages without ChemSpiderID
- Articles without KEGG source
- Articles without InChI source
- Articles without UNII source
- Articles containing unverified chemical infoboxes
- Amphetamine alkaloids
- Amphetamines
- Sympathomimetic amines
- Decongestants
- Cardiovascular Drugs
- Drug