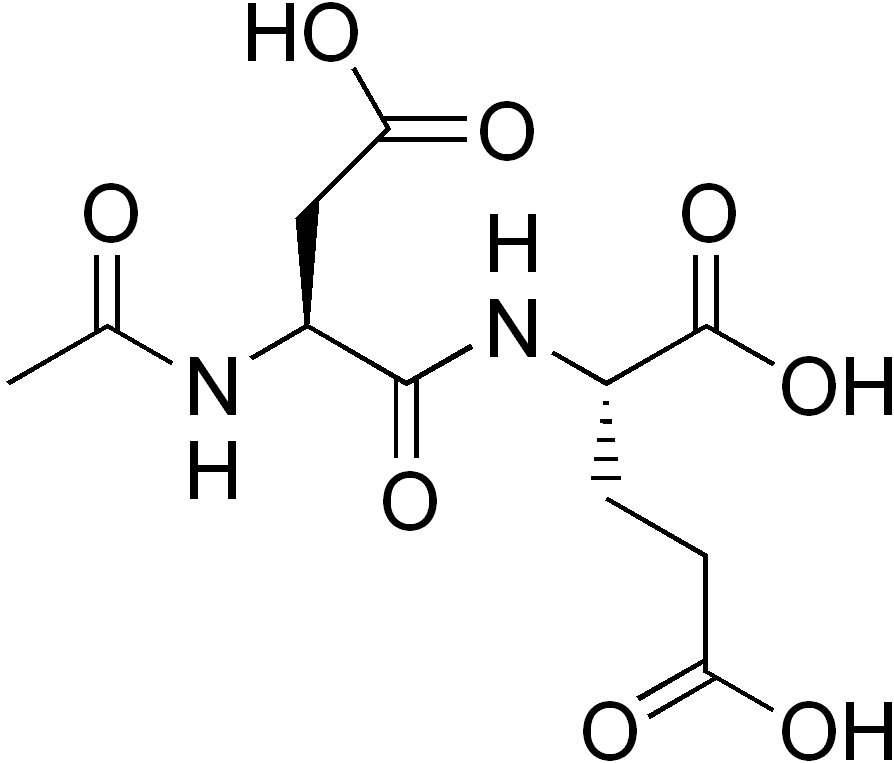
N-Acetylaspartylglutamate
| |
| Names | |
|---|---|
Other names
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | Lua error in Module:Wikidata at line 879: attempt to index field 'wikibase' (a nil value). Lua error in Module:Wikidata at line 879: attempt to index field 'wikibase' (a nil value). |
| MeSH | N-acetyl-1-aspartylglutamic+acid |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C11H16N2O8 | |
| Molar mass | 304.26 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]
Overview
N-Acetylaspartylglutamic acid (N-acetylaspartylglutamate or NAAG) is a peptide neurotransmitter and the third-most-prevalent neurotransmitter in the mammalian nervous system. NAAG consists of N-acetylaspartic acid (NAA) and glutamic acid coupled via a peptide bond. NAAG was discovered as a nervous system-specific peptide in 1965 by Curatolo and colleagues[2] but was not extensively studied. It meets the criteria for a neurotransmitter, including being concentrated in neurons, packed in synaptic vesicles, released in a calcium-dependent manner, and hydrolyzed in the synaptic space by enzymatic activity. NAAG activates a specific receptor, the metabotropic glutamate receptor type 3. It is synthesized enzymatically from its two precursors and catabolized by NAAG peptidases in the synapse. The inhibition of the latter enzymes has potentially important therapeutic effects in animal models of several neurologic conditions and disorders.
Under the INN spaglumic acid,[1] NAAG is used as an antiallergic medication in eye drops and nasal preparations.
Research history
After its discovery in 1965, NAAG was disregarded as a neurotransmitter for several reasons. First, neuropeptides were not considered neurotransmitters until years later. Second, it did not seem to directly affect membrane potential, so it was classified as a metabolic intermediate. The importance of brain peptides became clearer with the discovery of endogenous opioids. Whereas the ability of NAAG to interact with NMDA receptors in a manner relevant to physiology is controversial, its primary receptor was long believed to be the mGluR3. Its interaction with the mGluR3 causes an activation of G proteins that reduce the concentration of the second messengers cAMP and cGMP in the both nerve cells and glia. This can lead to several changes in the cellular activity, including regulation of gene expression, reduction in the release of transmitter, and inhibition of long-term potentiation.[3][4] Stimulation of the mGluR3 by NAAG has been, however, questioned, finding relevant glutamate contamination in commercially available NAAG.[5][6]
According to one publication, NAAG can be differentiated from NAA in vivo by MR spectroscopy at 3 Tesla.[7]
Biosynthesis
NAAG synthetase activity mediates the biosynthesis of NAAG from glutamate and NAA, but little is known about the mechanism or regulation of this enzyme, and no NAAG synthetase activity has been isolated in cell-free preparations. Since other neuropeptides and nearly all vertebrate peptides are synthesized by post-translational processing, NAAG synthtase activity is relatively unique. As with NAA, the synthesis of NAAG is primarily restricted to neurons, although glial cells also contain and synthesize this peptide. In vitro, NAAG synthesis appears to be regulated by the availability of its precursor, NAA. In addition, during differentiation of neuroblastoma cells, it has been shown that a protein kinase A (PKA) activator will increase the quantity of NAAG, while a protein kinase C (PKC) activator will decrease its concentration. This finding suggests that PKA and PKC have opposing regulatory effects on the NAAG synthetase enzyme.[8][9]
Catabolism
NAAG is catabolized via NAAG peptidase activity. Two enzymes with NAAG peptidase activity have been cloned, glutamate carboxypeptidase II and glutamate carboxypeptidase III. These enzymes mediate the hydrolysis of NAAG to NAA and glutamate. Their inhibition can produce therapeutic benefits. Two main types of inhibitors of this enzyme are known: compounds related to 2-(phosphonomethyl)pentanedioic acid (2-PMPA) and urea-based analogs of NAAG, including ZJ43, ZJ17, and ZJ11. In rat models, ZJ43 and 2-PMPA reduce perception of inflammatory and neuropathic pain when administered systemically, intracerebrally, or locally, suggesting that NAAG modulates neurotrasmission in pain circuits via mGlu3 receptors. The inhibition of NAAG hydrolysis increases the concentration of NAAG in the synaptic space analogous to the effects of SSRIs in increasing the concentration of serotonin. This elevated NAAG gives greater activation of presynaptic mGluR3 receptors, which decrease release of transmitter (glutamate) in the pain signaling pathways of the spinal cord and brain. In the case of traumatic brain injury, the injection of a NAAG peptidase inhibitor reduces neuron and astrocyte death in the hippocampus nearest the site of the injury. In a mouse model of amyotrophic lateral sclerosis (ALS), the chronic inhibition of NAAG peptidase activity delayed the onset of ALS symptoms and slowed the progress of the neuronal death. To model schizophrenia, animals were injected with phencyclidine (PCP) and, therefore, exhibited symptoms of the disorder, such as social withdrawal and motor responses. Upon injection with ZJ43, these behaviors were decreased, suggesting that an increase in NAAG in the synapse — and its subsequent activation of mGluR3 receptors — has potential as a co-therapy for schizophernia. In these cases, NAAG peptidase inhibition reduces the adverse effects in these disorders. Future research focuses on the role of NAAG in pain perception, brain injury, and schizophrenia while developing NAAG peptidase inhibitors with even greater ability to cross the blood–brain barrier.[10][11][12][13][14]
See also
References
- ↑ 1.0 1.1 Spaglumic Acid, drugs.com
- ↑ CURATOLO A, D ARCANGELO P, LINO A, BRANCATI A (1965). "Distribution Of N-Acetyl-Aspartic And N-Acetyl-Aspartyl-Glutamic Acids In Nervous Tissue". J. Neurochem. 12 (4): 339–42. doi:10.1111/j.1471-4159.1965.tb06771.x. PMID 14340686.
- ↑ Coyle, J.T. (1997). "The nagging question of the function of N-acetylaspartylglutamate". Neurobiology of Disease. 4 (3–4): 231–8. doi:10.1006/nbdi.1997.0153. PMID 9361299.
- ↑ Neale, J.H., Bzdega, T., Wroblewska, B. (2000). "N-Acetylaspartylglutamate: The Most Abundant Peptide Neurotransmitter in the Mammalian Central Nervous System". Journal of Neurochemistry. 75 (2): 443–452. doi:10.1046/j.1471-4159.2000.0750443.x. PMID 10899918.
- ↑ Fricker, A.-C., Mok, M.H.S., de la Flor, R., Shah, A.J., Woolley, M., Dawson, L.A., Kew, J.N.C. (2009). "Effects of N-acetylaspartylglutamate (NAAG) at group II mGluRs and NMDAR". Neuropharmacology. 56 (6–7): 1060–67. doi:10.1016/j.neuropharm.2009.03.002. PMID 19285517.
- ↑ Chopra, M., Yao, Y., Blake, T.J., Hampson, D.R., Johnson, E.C. (2009). "The neuroactive peptide N-acetylaspartylglutamate is not an agonist at the metabotropic glutamate receptor subtype 3 of metabotropic glutamate receptor". The Journal of Pharmacology and Experimental Therapeutics. 330 (1): 212–19. doi:10.1124/jpet.109.152553. PMID 19389924.
- ↑ Edden RA, Pomper MG, Barker PB (2007). "In vivo differentiation of N-acetyl aspartyl glutamate from N-acetyl aspartate at 3 Tesla". Magnetic resonance in medicine : official journal of the Society of Magnetic Resonance in Medicine / Society of Magnetic Resonance in Medicine. 57 (6): 977–82. doi:10.1002/mrm.21234. PMID 17534922.
- ↑ Gehl, Laura, Omar Saab, Tomasz Bzdega, Barbara Wroblewska and Joseph Neale (2004). "Biosynthesis of NAAG by an enzyme-mediate process in rat central nervous neurons and glia". Journal of Neurochemistry. 90 (4): 989–997. doi:10.1111/j.1471-4159.2004.02578.x. PMID 15287905.
- ↑ Arun, P., Madhavarao, C.N., Moffett, J.R., Namboodiri, M.A.A. (2006). "Regulation of N-acetylaspartate and N-acetylaspartylglutamate biosynthesis by protein kinase activators". Journal of Neurochemistry. 98 (6): 2034–2042. doi:10.1111/j.1471-4159.2006.04068.x. PMID 16945114.
- ↑ Riveros, N.; Orrego, F. (1984). "A study of possible excitatory effects of N-acetylaspartylglutamate in different in vivo and in vitro brain preparations". Brain Research. 299 (2): 393–395. doi:10.1016/0006-8993(84)90727-3. PMID 6145497.
- ↑ Yamamoto, T., Saito, O., Aoe, T., Bartolozzi, A., Sarva, J., Kozikowski, A., Wroblewska, B., Bzdega, T., and Neale, J. H. (2007). "Local Administration of N-Acetylaspartylglutamate (NAAG) Peptidase Inhibitors Is Analgesic in Peripheral Pain". European Journal of Neuroscience. 25 (1): 147–158. doi:10.1111/j.1460-9568.2006.05272.x. PMID 17241276.
- ↑ Zhou, J, Neale, J.H, Pomper,M.G., Kozikowski, A.P (2005). "NAAG Peptidase Inhibitors and their Potential for Diagnosis and Therapy". Nature Reviews Drug Discovery. 4 (12): 1015–1026. doi:10.1038/nrd1903. PMID 16341066.
- ↑ Neale, J.H., Olszewski, R.T., Gehl, L.M., Wroblewska, B., Bzdega, T. (2005). "The neurotransmitter N-acetylaspartyl-glutamate in models of pain, ALS, diabetic neuropathy, CNS injury and schizophrenia". Trends in Pharmacological Sciences. 26 (9): 477–484. doi:10.1016/j.tips.2005.07.004. PMID 16055199.
- ↑ Olszewski, R.T., Wegorzewska, M.M., Monteiro, A.C., Krolikowski, K.A., Zhou, J., Kozikowski, A.P., Long, K., Mastropaolo, J., Deutsch, S.I. and Neale, J.H. (2007). "Phencyclidine and Dizocilpine Induced Behaviors Reduced by N-acetylaspartylglutamate Peptidase Inhibition via Metabotropic Glutamate Receptors". Biological Psychiatry. doi:10.1016/j.biopsych.2007.04.016.
- Pages with ignored display titles
- Pages with script errors
- CS1 maint: Multiple names: authors list
- Articles without InChI source
- Articles without EBI source
- Articles without KEGG source
- Chemical articles with unknown parameter in Chembox
- Articles with changed CASNo identifier
- ECHA InfoCard ID from Wikidata
- Articles with changed FDA identifier
- Articles containing unverified chemical infoboxes
- Chembox image size set
- Neuropeptides
- Drug