Desogestrel and Ethinyl Estradiol
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Alberto Plate [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Desogestrel and Ethinyl Estradiol is an oral contraceptive that is FDA approved for the prophylaxis of unplanned pregnancy in women who elect to use oral contraceptives as a method of contraception. Common adverse reactions include cholasma, weight variations, bloating, nausea, vomiting, cramps, migraine, depression, amenorrhea, bleeding, nipple discharge, menstruations disorders, vaginal bleeding and breast swelling.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
To achieve maximum contraceptive effectiveness, Apri must be taken exactly as directed and at intervals not exceeding 24 hours. Apri is available in the tablet dispenser which is preset for a Sunday Start. Day 1 Start is also provided.
Day 1 Start
- The dosage of Apri for the initial cycle of therapy is one rose-colored “active” tablet administered daily from the 1st day through the 21st day of the menstrual cycle, counting the first day of menstrual flow as “Day 1”. Tablets are taken without interruption as follows: One rose-colored “active” tablet daily for 21 days, then one white “reminder” tablet daily for 7 days. After 28 tablets have been taken, a new course is started and a rose-colored “active” tablet is taken the next day.
- The use of Apri for contraception may be initiated 4 weeks postpartum in women who elect not to breastfeed. When the tablets are administered during the postpartum period, the increased risk of thromboembolic disease associated with the postpartum period must be considered. If the patient starts on Apri postpartum, and has not yet had a period, she should be instructed to use another method of contraception until a rose-colored “active” tablet has been taken daily for 7 days. The possibility of ovulation and conception prior to initiation of medication should be considered. If the patient misses one (1) rose-colored “active” tablet in Weeks 1, 2, or 3, the rose-colored “active” tablet should be taken as soon as she remembers. If the patient misses two (2) rose-colored “active” tablets in Week 1 or Week 2, the patient should take two (2) rose-colored “active” tablets the day she remembers and two (2) rose-colored “active” tablets the next day; and then continue taking one (1) rose-colored “active” tablet a day until she finishes the pack. The patient should be instructed to use a back-up method of birth control such as a condom or spermicide if she has sex in the seven (7) days after missing pills. If the patient misses two (2) rose-colored “active” tablets in the third week or misses three (3) or more rose-colored “active” tablets in a row, the patient should throw out the rest of the pack and start a new pack that same day. The patient should be instructed to use a back-up method of birth control if she has sex in the seven (7) days after missing pills.
Sunday Start
- When taking Apri, the first rose-colored “active” tablet should be taken on the first Sunday after menstruation begins. If the period begins on Sunday, the first rose-colored “active” tablet is taken on that day. If switching directly from another oral contraceptive, the first rose-colored “active” tablet should be taken on the first Sunday after the last ACTIVE tablet of the previous product. Tablets are taken without interruption as follows: One rose-colored “active” tablet daily for 21 days, then one white “reminder” tablet daily for 7 days. After 28 tablets have been taken, a new course is started and a rose-colored “active” tablet is taken the next day (Sunday). When initiating a Sunday start regimen, another method of contraception should be used until after the first 7 consecutive days of administration.
- The use of Apri for contraception may be initiated 4 weeks postpartum. When the tablets are administered during the postpartum period, the increased risk of thromboembolic disease associated with the postpartum period must be considered. If the patient starts on Apri postpartum, and has not yet had a period, she should be instructed to use another method of contraception until a rose-colored “active” tablet has been taken daily for 7 days. The possibility of ovulation and conception prior to initiation of medication should be considered. If the patient misses one (1) rose-colored “active” tablet in Weeks 1, 2, or 3, the rose-colored “active” tablet should be taken as soon as she remembers. If the patient misses two (2) rose-colored “active” tablets in Week 1 or Week 2, the patient should take two (2) rose-colored “active” tablets the day she remembers and two (2) rose-colored “active” tablets the next day; and then continue taking one (1) rose-colored “active” tablet a day until she finishes the pack. The patient should be instructed to use a back-up method of birth control such as a condom or spermicide if she has sex in the seven (7) days after missing pills. If the patient misses two (2) rose-colored “active” tablets in the third week or misses three (3) or more rose-colored “active” tablets in a row, the patient should continue taking one rose-colored “active” tablet every day until Sunday. On Sunday the patient should throw out the rest of the pack and start a new pack that same day. The patient should be instructed to use a back-up method of birth control if she has sex in the seven (7) days after missing pills.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Desogestrel and Ethinyl Estradiol in adult patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Desogestrel and Ethinyl Estradiol in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
To achieve maximum contraceptive effectiveness, Apri must be taken exactly as directed and at intervals not exceeding 24 hours. Apri is available in the tablet dispenser which is preset for a Sunday Start. Day 1 Start is also provided.
Day 1 Start
- The dosage of Apri for the initial cycle of therapy is one rose-colored “active” tablet administered daily from the 1st day through the 21st day of the menstrual cycle, counting the first day of menstrual flow as “Day 1”. Tablets are taken without interruption as follows: One rose-colored “active” tablet daily for 21 days, then one white “reminder” tablet daily for 7 days. After 28 tablets have been taken, a new course is started and a rose-colored “active” tablet is taken the next day.
- The use of Apri for contraception may be initiated 4 weeks postpartum in women who elect not to breastfeed. When the tablets are administered during the postpartum period, the increased risk of thromboembolic disease associated with the postpartum period must be considered. If the patient starts on Apri postpartum, and has not yet had a period, she should be instructed to use another method of contraception until a rose-colored “active” tablet has been taken daily for 7 days. The possibility of ovulation and conception prior to initiation of medication should be considered. If the patient misses one (1) rose-colored “active” tablet in Weeks 1, 2, or 3, the rose-colored “active” tablet should be taken as soon as she remembers. If the patient misses two (2) rose-colored “active” tablets in Week 1 or Week 2, the patient should take two (2) rose-colored “active” tablets the day she remembers and two (2) rose-colored “active” tablets the next day; and then continue taking one (1) rose-colored “active” tablet a day until she finishes the pack. The patient should be instructed to use a back-up method of birth control such as a condom or spermicide if she has sex in the seven (7) days after missing pills. If the patient misses two (2) rose-colored “active” tablets in the third week or misses three (3) or more rose-colored “active” tablets in a row, the patient should throw out the rest of the pack and start a new pack that same day. The patient should be instructed to use a back-up method of birth control if she has sex in the seven (7) days after missing pills.
Sunday Start
- When taking Apri, the first rose-colored “active” tablet should be taken on the first Sunday after menstruation begins. If the period begins on Sunday, the first rose-colored “active” tablet is taken on that day. If switching directly from another oral contraceptive, the first rose-colored “active” tablet should be taken on the first Sunday after the last ACTIVE tablet of the previous product. Tablets are taken without interruption as follows: One rose-colored “active” tablet daily for 21 days, then one white “reminder” tablet daily for 7 days. After 28 tablets have been taken, a new course is started and a rose-colored “active” tablet is taken the next day (Sunday). When initiating a Sunday start regimen, another method of contraception should be used until after the first 7 consecutive days of administration.
- The use of Apri for contraception may be initiated 4 weeks postpartum. When the tablets are administered during the postpartum period, the increased risk of thromboembolic disease associated with the postpartum period must be considered. If the patient starts on Apri postpartum, and has not yet had a period, she should be instructed to use another method of contraception until a rose-colored “active” tablet has been taken daily for 7 days. The possibility of ovulation and conception prior to initiation of medication should be considered. If the patient misses one (1) rose-colored “active” tablet in Weeks 1, 2, or 3, the rose-colored “active” tablet should be taken as soon as she remembers. If the patient misses two (2) rose-colored “active” tablets in Week 1 or Week 2, the patient should take two (2) rose-colored “active” tablets the day she remembers and two (2) rose-colored “active” tablets the next day; and then continue taking one (1) rose-colored “active” tablet a day until she finishes the pack. The patient should be instructed to use a back-up method of birth control such as a condom or spermicide if she has sex in the seven (7) days after missing pills. If the patient misses two (2) rose-colored “active” tablets in the third week or misses three (3) or more rose-colored “active” tablets in a row, the patient should continue taking one rose-colored “active” tablet every day until Sunday. On Sunday the patient should throw out the rest of the pack and start a new pack that same day. The patient should be instructed to use a back-up method of birth control if she has sex in the seven (7) days after missing pills.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Desogestrel and Ethinyl Estradiol in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Desogestrel and Ethinyl Estradiol in pediatric patients.
Contraindications
Oral contraceptives should not be used in women who currently have the following conditions:
- Thrombophlebitis or thromboembolic disorders
- A past history of deep vein thrombophlebitis or thromboembolic disorders
- Known thrombophilic conditions
- Cerebral vascular disease or coronary artery disease (current or history)
- Valvular heart disease with complications
- Persistent blood pressure values of > 160 mm Hg systolic or > 100 mg Hg diastolic
- Diabetes with vascular involvement
- Headaches with focal neurological symptoms
- Major surgery with prolonged immobilization
- Known or suspected carcinoma of the breast or personal history of breast cancer
- Endometrial carcinoma or other known or suspected estrogen-dependent neoplasia
- Undiagnosed abnormal genital bleeding
- Cholestatic jaundice of pregnancy or jaundice with prior pill use
- Acute or chronic hepatocellular disease with abnormal liver function
- Hepatic adenomas or carcinomas
- Known or suspected pregnancy
- Hypersensitivity to any component of this product
Warnings
Cigarette smoking increases the risk of serious cardiovascular events from combination oral contraceptive use. This risk increases with age, particularly in women over 35 years of age, and with the number of cigarettes smoked. For this reason, combination oral contraceptives, including Apri®, should not be used by women who are over 35 years of age and smoke.
The use of oral contraceptives is associated with increased risks of several serious conditions including myocardial infarction, thromboembolism, stroke, hepatic neoplasia, and gallbladder disease, although the risk of serious morbidity or mortality is very small in healthy women without underlying risk factors. The risk of morbidity and mortality increases significantly in the presence of other underlying risk factors such as hypertension, hyperlipidemias, obesity and diabetes.
Practitioners prescribing oral contraceptives should be familiar with the following information relating to these risks.
The information contained in this package insert is principally based on studies carried out in patients who used oral contraceptives with formulations of higher doses of estrogens and progestogens than those in common use today. The effect of long term use of the oral contraceptives with formulations of lower doses of both estrogens and progestogens remains to be determined.
Throughout this labeling, epidemiological studies reported are of two types: retrospective or case control studies and prospective or cohort studies. Case control studies provide a measure of the relative risk of a disease, namely, a ratio of the incidence of a disease among oral contraceptive users to that among nonusers. The relative risk does not provide information on the actual clinical occurrence of a disease. Cohort studies provide a measure of attributable risk, which is the difference in the incidence of disease between oral contraceptive users and nonusers. The attributable risk does provide information about the actual occurrence of a disease in the population (Adapted from refs. 2 and 3 with the author’s permission). For further information, the reader is referred to a text on epidemiological methods.
Thromboembolic Disorder and Other Vascular Problems
Thromboembolism
- An increased risk of thromboembolic and thrombotic disease associated with the use of oral contraceptives is well established. Case control studies have found the relative risk of users compared to nonusers to be 3 for the first episode of superficial venous thrombosis, 4 to 11 for deep vein thrombosis or pulmonary embolism, and 1.5 to 6 for women with predisposing conditions for venous thromboembolic disease. Cohort studies have shown the relative risk to be somewhat lower, about 3 for new cases and about 4.5 for new cases requiring hospitalization 25. The risk of thromboembolic disease associated with oral contraceptives is not related to length of use and disappears after pill use is stopped. Several epidemiologic studies indicate that third generation oral contraceptives, including those containing desogestrel, are associated with a higher risk of venous thromboembolism than certain second generation oral contraceptives. In general, these studies indicate an approximate 2-fold increased risk, which corresponds to an additional 1 to 2 cases of venous thromboembolism per 10,000 women-years of use. However, data from additional studies have not shown this 2-fold increase in risk.
- A two- to four-fold increase in relative risk of post-operative thromboembolic complications has been reported with the use of oral contraceptives. The relative risk of venous thrombosis in women who have predisposing conditions is twice that of women without such medical conditions. If feasible, oral contraceptives should be discontinued at least four weeks prior to and for two weeks after elective surgery of a type associated with an increase in risk of thromboembolism and during and following prolonged immobilization. Since the immediate postpartum period is also associated with an increased risk of thromboembolism, oral contraceptives should be started no earlier than four weeks after delivery in women who elect not to breastfeed.
Myocardial Infarction
- An increased risk of myocardial infarction has been attributed to oral contraceptive use. This risk is primarily in smokers or women with other underlying risk factors for coronary artery disease such as hypertension, hypercholesterolemia, morbid obesity, and diabetes. The relative risk of heart attack for current oral contraceptive users has been estimated to be two to six4 to 10. The risk is very low in women under the age of 30.
- Smoking in combination with oral contraceptive use has been shown to contribute substantially to the incidence of myocardial infarctions in women in their mid-thirties or older with smoking accounting for the majority of excess cases. Mortality rates associated with circulatory disease have been shown to increase substantially in smokers, especially in those 35 years of age and older and in nonsmokers over the age of 40 among women who use oral contraceptives.
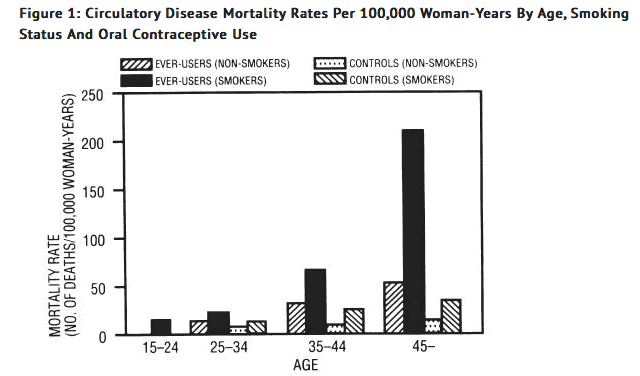
- Oral contraceptives may compound the effects of well-known risk factors, such as hypertension, diabetes, hyperlipidemias, age and obesity. In particular, some progestogens are known to decrease HDL cholesterol and cause glucose intolerance, while estrogens may create a state of hyperinsulinism. Oral contraceptives have been shown to increase blood pressure among users. Similar effects on risk factors have been associated with an increased risk of heart disease. Oral contraceptives must be used with caution in women with cardiovascular risk factors.
- There is some evidence that the risk of myocardial infarction associated with oral contraceptives is lower when the progestogen has minimal androgenic activity than when the activity is greater. Receptor binding and animal studies have shown that desogestrel or its active metabolite has minimal androgenic activity, although these findings have not been confirmed in adequate and well-controlled clinical trials.
Cerebrovascular Diseases
- Oral contraceptives have been shown to increase both the relative and attributable risks of cerebrovascular events (thrombotic strokes and hemorrhagic strokes), although, in general, the risk is greatest among older (> 35 years), hypertensive women who also smoke. Hypertension was found to be a risk factor for both users and nonusers, for both types of strokes, and smoking interacted to increase the risk of stroke 27 to 29.
- In a large study, the relative risk of thrombotic strokes has been shown to range from 3 for normotensive users to 14 for users with severe hypertension. The relative risk of hemorrhagic stroke is reported to be 1.2 for non-smokers who used oral contraceptives, 2.6 for smokers who did not use oral contraceptives, 7.6 for smokers who used oral contraceptives, 1.8 for normotensive users and 25.7 for users with severe hypertension. The attributable risk is also greater in older women.
Dose-Related Risk of Vascular Disease from Oral Contraceptives
- A positive association has been observed between the amount of estrogen and progestogen in oral contraceptives and the risk of vascular disease. A decline in serum high density lipoproteins (HDL) has been reported with many progestational agents. A decline in serum high density lipoproteins has been associated with an increased incidence of ischemic heart disease. Because estrogens increase HDL cholesterol, the net effect of an oral contraceptive depends on a balance achieved between doses of estrogen and progestogen and the nature and absolute amount of progestogens used in the contraceptives. The amount of both hormones should be considered in the choice of an oral contraceptive.
- Minimizing exposure to estrogen and progestogen is in keeping with good principles of therapeutics. For any particular estrogen/progestogen combination, the dosage regimen prescribed should be one which contains the least amount of estrogen and progestogen that is compatible with a low failure rate and the needs of the individual patient. New acceptors of oral contraceptive agents should be started on preparations containing the lowest estrogen content which is judged appropriate for the individual patient.
Persistence of Risk of Vascular Disease
- There are two studies which have shown persistence of risk of vascular disease for ever-users of oral contraceptives. In a study in the United States, the risk of developing myocardial infarction after discontinuing oral contraceptives persists for at least 9 years for women 40 to 49 years old who had used oral contraceptives for five or more years, but this increased risk was not demonstrated in other age groups. In another study in Great Britain, the risk of developing cerebrovascular disease persisted for at least 6 years after discontinuation of oral contraceptives, although excess risk was very small. However, both studies were performed with oral contraceptive formulations containing 0.050 mg or higher of estrogens.
Estimates of Mortality from Contraceptive Use
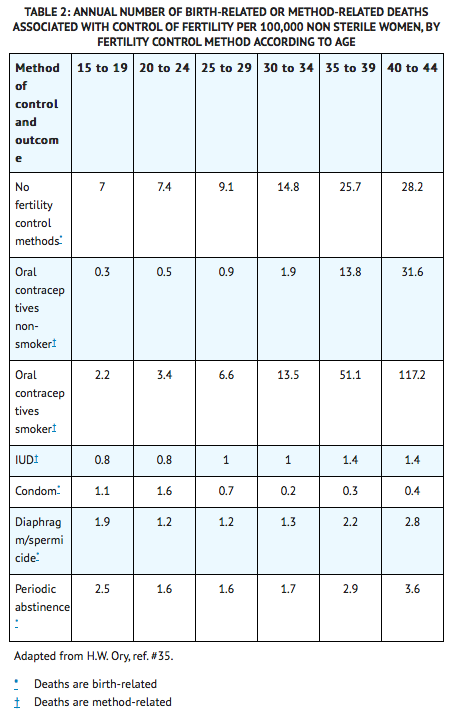
- One study gathered data from a variety of sources which have estimated the mortality rate associated with different methods of contraception at different ages (Table 2). These estimates include the combined risk of death associated with contraceptive methods plus the risk attributable to pregnancy in the event of method failure. Each method of contraception has its specific benefits and risks. The study concluded that with the exception of oral contraceptive users 35 and older who smoke and 40 and older who do not smoke, mortality associated with all methods of birth control is low and below that associated with childbirth.
- The observation of an increase in risk of mortality with age for oral contraceptive users is based on data gathered in the 1970’s35. Current clinical recommendation involves the use of lower estrogen dose formulations and a careful consideration of risk factors. In 1989, the Fertility and Maternal Health Drugs Advisory Committee was asked to review the use of oral contraceptives in women 40 years of age and over. The Committee concluded that although cardiovascular disease risk may be increased with oral contraceptive use after age 40 in healthy non-smoking women (even with the newer low-dose formulations), there are also greater potential health risks associated with pregnancy in older women and with the alternative surgical and medical procedures which may be necessary if such women do not have access to effective and acceptable means of contraception. The Committee recommended that the benefits of low-dose oral contraceptive use by healthy non-smoking women over 40 may outweigh the possible risks.
- Of course, older women, as all women who take oral contraceptives, should take an oral contraceptive which contains the least amount of estrogen and progestogen that is compatible with a low failure rate and individual patient needs.
Carcinoma of the Reproductive Organs and Breasts
- Numerous epidemiological studies have been performed on the incidence of breast cancer, endometrial cancer, ovarian cancer, and cervical cancer in women using oral contraceptives. The risk of having breast cancer diagnosed may be slightly increased among current and recent users of combined oral contraceptives (COC). However, this excess risk appears to decrease over time after COC discontinuation and by 10 years after cessation the increased risk disappears. Some studies report an increased risk with duration of use while other studies do not and no consistent relationships have been found with dose or type of steroid. Some studies have found a small increase in risk for women who first use COC before age 20. Most studies show a similar pattern of risk with COC use regardless of a woman’s reproductive history or her family breast cancer history. Breast cancers diagnosed in current or previous oral contraceptive users tend to be less clinically advanced than in nonusers. Women who currently have or have had breast cancer should not use oral contraceptives because breast cancer is usually a hormonally-sensitive tumor.
- Some studies suggest that oral contraceptive use has been associated with an increase in the risk of cervical intraepithelial neoplasia in some populations of women. However, there continues to be controversy about the extent to which such findings may be due to differences in sexual behavior and other factors. In spite of many studies of the relationship between oral contraceptive use and breast and cervical cancers, a cause-and-effect relationship has not been established.
Hepatic Neoplasia
- Benign hepatic adenomas are associated with oral contraceptive use, although the incidence of benign tumors is rare in the United States. Indirect calculations have estimated the attributable risk to be in the range of 3.3 cases/100,000 for users, a risk that increases after four or more years of use especially with oral contraceptives of higher dose. Rupture of benign, hepatic adenomas may cause death through intra-abdominal hemorrhage.
- Studies from Britain have shown an increased risk of developing hepatocellular carcinoma in long-term (> 8 years) oral contraceptive users. However, these cancers are extremely rare in the U.S. and the attributable risk (the excess incidence) of liver cancers in oral contraceptive users approaches less than one per million users.
Ocular Lesions
- There have been clinical case reports of retinal thrombosis associated with the use of oral contraceptives. Oral contraceptives should be discontinued if there is unexplained partial or complete loss of vision; onset of proptosis or diplopia; papilledema; or retinal vascular lesions. Appropriate diagnostic and therapeutic measures should be undertaken immediately.
Oral Contraceptive Use Before or During Early Pregnancy
- Extensive epidemiological studies have revealed no increased risk of birth defects in women who have used oral contraceptives prior to pregnancy. The majority of recent studies also do not indicate a teratogenic effect, particularly in so far as cardiac anomalies and limb reduction defects are concerned, when oral contraceptives are taken inadvertently during early pregnancy.
- The administration of oral contraceptives to induce withdrawal bleeding should not be used as a test for pregnancy. Oral contraceptives should not be used during pregnancy to treat threatened or habitual abortion.
- It is recommended that for any patient who has missed two consecutive periods, pregnancy should be ruled out. If the patient has not adhered to the prescribed schedule, the possibility of pregnancy should be considered at the time of the first missed period. Oral contraceptive use should be discontinued if pregnancy is confirmed.
Gallbladder Disease
- Earlier studies have reported an increased lifetime relative risk of gallbladder surgery in users of oral contraceptives and estrogens. More recent studies, however, have shown that the relative risk of developing gallbladder disease among oral contraceptive users may be minimal. The recent findings of minimal risk may be related to the use of oral contraceptive formulations containing lower hormonal doses of estrogens and progestogens.
Carbohydrate and Lipid Metabolic Effects
- Oral contraceptives have been shown to cause a decrease in glucose tolerance in a significant percentage of users. This effect has been shown to be directly related to estrogen dose. In general, progestogens increase insulin secretion and create insulin resistance, this effect varying with different progestational agents. In the nondiabetic woman, oral contraceptives appear to have no effect on fasting blood glucose. Because of these demonstrated effects, prediabetic and diabetic women should be carefully monitored while taking oral contraceptives.
- A small proportion of women will have persistent hypertriglyceridemia while on the pill. As discussed earlier, changes in serum triglycerides and lipoprotein levels have been reported in oral contraceptive users.
Elevated Blood Pressure
- Women with significant hypertension should not be started on hormonal contraception. An increase in blood pressure has been reported in women taking oral contraceptives and this increase is more likely in older oral contraceptive users and with extended duration of use. Data from the Royal College of General Practitioners and subsequent randomized trials have shown that the incidence of hypertension increases with increasing progestational activity and concentrations of progestogens.
- Women with a history of hypertension or hypertension-related diseases, or renal disease should be encouraged to use another method of contraception. If these women elect to use oral contraceptives, they should be monitored closely and if a clinically significant persistent elevation of blood pressure (BP) occurs (≥ 160 mm Hg systolic or ≥ 100 mm Hg diastolic) and cannot be adequately controlled, oral contraceptives should be discontinued. In general, women who develop hypertension during hormonal contraceptive therapy should be switched to a non-hormonal contraceptive. If other contraceptive methods are not suitable, hormonal contraceptive therapy may continue combined with antihypertensive therapy. Regular monitoring of BP throughout hormonal contraceptive therapy is recommended. For most women, elevated blood pressure will return to normal after stopping oral contraceptives, and there is no difference in the occurrence of hypertension among former and never users.
Headache
- The onset or exacerbation of migraine or development of headache with a new pattern which is recurrent, persistent or severe requires discontinuation of oral contraceptives and evaluation of the cause.
Bleeding Irregularities
- Breakthrough bleeding and spotting are sometimes encountered in patients on oral contraceptives, especially during the first three months of use. Nonhormonal causes should be considered and adequate diagnostic measures taken to rule out malignancy or pregnancy in the event of breakthrough bleeding, as in the case of any abnormal vaginal bleeding. If pathology has been excluded, time or a change to another formulation may solve the problem. In the event of amenorrhea, pregnancy should be ruled out. Some women may encounter post-pill amenorrhea or oligomenorrhea, especially when such a condition was pre-existent.
Ectopic Pregnancy
- Ectopic as well as intrauterine pregnancy may occur in contraceptive failures.
Adverse Reactions
Clinical Trials Experience
An increased risk of the following serious adverse reactions has been associated with the use of oral contraceptives:
- Thrombophlebitis and venous thrombosis with or without embolism
- Arterial thromboembolism
- Pulmonary embolism
- Myocardial infarction
- Cerebral hemorrhage
- Cerebral thrombosis
- Hypertension
- Gallbladder disease
- Hepatic adenomas or benign liver tumors
There is evidence of an association between the following conditions and the use of oral contraceptives:
The following adverse reactions have been reported in patients receiving oral contraceptives and are believed to be drug-related:
- Nausea
- Vomiting
- Gastrointestinal symptoms (such as abdominal cramps and bloating)
- Breakthrough bleeding
- Spotting
- Change in menstrual flow
- Amenorrhea
- Temporary infertility after discontinuation of treatment
- Edema
- Melasma which may persist
- Breast changes: tenderness, enlargement, secretion
- Change in weight (increase or decrease)
- Change in cervical erosion and secretion
- Diminution in lactation when given immediately postpartum
- Cholestatic jaundice
- Migraine
- Allergic reaction, including rash, urticaria, and angioedema
- Mental depression
- Reduced tolerance to carbohydrates
- Vaginal candidiasis
- Change in corneal curvature (steepening)
- Intolerance to contact lenses
The following adverse reactions have been reported in users of oral contraceptives and a causal association has been neither confirmed nor refuted:
- Pre-menstrual syndrome
- Cataracts
- Changes in appetite
- Cystitis-like syndrome
- Headache
- Nervousness
- Dizziness
- Hirsutism
- Loss of scalp hair
- Erythema multiforme
- Erythema nodosum
- Hemorrhagic eruption
- Vaginitis
- Porphyria
- Impaired renal function
- Hemolytic uremic syndrome
- Acne
- Changes in libido
- Colitis
- Budd-Chiari Syndrome
Postmarketing Experience
There is limited information regarding Desogestrel and Ethinyl Estradiol Postmarketing Experience in the drug label.
Drug Interactions
Substances decreasing the plasma concentrations of COCs and potentially diminishing the efficacy of COCs
- Drugs or herbal products that induce certain enzymes, including cytochrome P450 3A4 (CYP3A4), may decrease the plasma concentrations of COCs and potentially diminish the effectiveness of CHCs or increase breakthrough bleeding. Some drugs or herbal products that may decrease the effectiveness of hormonal contraceptives include phenytoin, barbiturates, carbamazepine, bosentan, felbamate, griseofulvin, oxcarbazepine, rifampicin, topiramate, rifabutin, rufinamide, aprepitant, and products containing St. John’s wort. Interactions between hormonal contraceptives and other drugs may lead to breakthrough bleeding and/or contraceptive failure. Counsel women to use an alternative method of contraception or a back-up method when enzyme inducers are used with CHCs, and to continue back-up contraception for 28 days after discontinuing the enzyme inducer to ensure contraceptive reliability.
Substances increasing the plasma concentrations of COCs
- Coadministration of atorvastatin or rosuvastatin and certain COCs containing EE increase AUC values for EE by approximately 20 to 25%. Ascorbic acid and acetaminophen may increase plasma EE concentrations, possibly by inhibition of conjugation. CYP3A4 inhibitors such as itraconazole, voriconazole, fluconazole, grapefruit juice, or ketoconazole may increase plasma hormone concentrations.
Human immunodeficiency virus (HIV)/Hepatitis C virus (HCV) protease inhibitors and non-nucleoside reverse transcriptase inhibitors
- Significant changes (increase or decrease) in the plasma concentrations of estrogen and/or progestin have been noted in some cases of coadministration with HIV protease inhibitors (decrease e.g., nelfinavir, ritonavir, darunavir/ritonavir, (fos)amprenavir/ritonavir, lopinavir/ritonavir, and tipranavir/ritonavir or increase e.g., indinavir and atazanavir/ritonavir) /HCV protease inhibitors (decrease e.g., boceprevir and telaprevir) or with non-nucleoside reverse transcriptase inhibitors (decrease e.g., nevirapine or increase e.g., etravirine).
Colesevelam: Colesevelam, a bile acid sequestrant, given together with a combination oral hormonal contraceptive, has been shown to significantly decrease the AUC of EE. A drug interaction between the contraceptive and colesevelam was decreased when the two drug products were given 4 hours apart.
Effects of Combined Hormonal Contraceptives on Other Drugs
- COCs containing EE may inhibit the metabolism of other compounds (e.g., cyclosporine, prednisolone, theophylline, tizanidine, and voriconazole) and increase their plasma concentrations. COCs have been shown to decrease plasma concentrations of acetaminophen, clofibric acid, morphine, salicylic acid, temazepam and lamotrigine. Significant decrease in plasma concentration of lamotrigine has been shown, likely due to induction of lamotrigine glucuronidation. This may reduce seizure control; therefore, dosage adjustments of lamotrigine may be necessary.
- Women on thyroid hormone replacement therapy may need increased doses of thyroid hormone because serum concentrations of thyroid-binding globulin increases with use of COCs.
Use in Specific Populations
Pregnancy
Pregnancy Category (FDA): X
There is no FDA guidance on usage of Desogestrel and Ethinyl Estradiol in women who are pregnant.
Pregnancy Category (AUS):
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Desogestrel and Ethinyl Estradiol in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Desogestrel and Ethinyl Estradiol during labor and delivery.
Nursing Mothers
- Small amounts of oral contraceptive steroids have been identified in the milk of nursing mothers and a few adverse effects on the child have been reported, including jaundice and breast enlargement. In addition, oral contraceptives given in the postpartum period may interfere with lactation by decreasing the quantity and quality of breast milk. If possible, the nursing mother should be advised not to use oral contraceptives but to use other forms of contraception until she has completely weaned her child.
Pediatric Use
- Safety and efficacy of Apri tablets have been established in women of reproductive age. Safety and efficacy are expected to be the same for postpubertal adolescents under the age of 16 and for users 16 years and older. Use of this product before menarche is not indicated.
Geriatic Use
- This product has not been studied in women over 65 years of age and is not indicated in this population.
Gender
There is no FDA guidance on the use of Desogestrel and Ethinyl Estradiol with respect to specific gender populations.
Race
There is no FDA guidance on the use of Desogestrel and Ethinyl Estradiol with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Desogestrel and Ethinyl Estradiol in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Desogestrel and Ethinyl Estradiol in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Desogestrel and Ethinyl Estradiol in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Desogestrel and Ethinyl Estradiol in patients who are immunocompromised.
Administration and Monitoring
Administration
There is limited information regarding Desogestrel and Ethinyl Estradiol Administration in the drug label.
Monitoring
There is limited information regarding Desogestrel and Ethinyl Estradiol Monitoring in the drug label.
IV Compatibility
There is limited information regarding the compatibility of Desogestrel and Ethinyl Estradiol and IV administrations.
Overdosage
- Serious ill effects have not been reported following acute ingestion of large doses of oral contraceptives by young children. Overdosage may cause nausea, and withdrawal bleeding may occur in females.
Pharmacology
| |
Desogestrel and Ethinyl Estradiol
| |
| Systematic (IUPAC) name | |
| 13-ethyl-17-ethynyl- 11-methylidene- 1,2,3,6,7,8,9,10,12,13,14,15, 16,17- tetradecahydrocyclopenta[a] phenanthren-17-ol | |
| Identifiers | |
| CAS number | |
| ATC code | G03 |
| PubChem | |
| DrugBank | |
| Chemical data | |
| Formula | Template:OrganicBox atomTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox |
| Mol. mass | 310.473 g/mol |
| SMILES | & |
| Pharmacokinetic data | |
| Bioavailability | ? |
| Protein binding | 98.3% |
| Metabolism | ? |
| Half life | ? |
| Excretion | ? |
| Therapeutic considerations | |
| Pregnancy cat. |
X(US) |
| Legal status |
[[Prescription drug|Template:Unicode-only]](US) |
| Routes | oral |
Mechanism of Action
- Combined oral contraceptives act by suppression of gonadotropins. Although the primary mechanism of this action is inhibition of ovulation, other alterations include changes in the cervical mucus, which increase the difficulty of sperm entry into the uterus, and changes in the endometrium which reduce the likelihood of implantation.
Structure
There is limited information regarding Desogestrel and Ethinyl Estradiol Structure in the drug label.
Pharmacodynamics
- Combined oral contraceptives act by suppression of gonadotropins. Although the primary mechanism of this action is inhibition of ovulation, other alterations include changes in the cervical mucus, which increase the difficulty of sperm entry into the uterus, and changes in the endometrium which reduce the likelihood of implantation.
- Receptor binding studies, as well as studies in animals, have shown that 3-keto-desogestrel, the biologically active metabolite of desogestrel, combines high progestational activity with minimal intrinsic androgenicity 91,92. The relevance of this latter finding in humans is unknown.
Pharmacokinetics
- Desogestrel is rapidly and almost completely absorbed and converted into 3-keto-desogestrel, its biologically active metabolite. Following oral administration, the relative bioavailability of desogestrel, as measured by serum levels of 3-keto-desogestrel, is approximately 84%.
- In the third cycle of use after a single dose of Apri, maximum concentrations of 3-keto-desogestrel of 2,805 ± 1,203 pg/mL (mean ± SD) are reached at 1.4 ± 0.8 hours. The area under the curve (AUC0 to ∞) is 33,858 ± 11,043 pg/mL • hr after a single dose. At steady state, attained from at least day 19 onwards, maximum concentrations of 5,840 ± 1,667 pg/mL are reached at 1.4 ± 0.9 hours. The minimum plasma levels of 3-keto-desogestrel at steady state are 1,400 ± 560 pg/mL. The AUC0 to 24 at steady state is 52,299 ± 17,878 pg/mL • hr. The mean AUC0 to ∞ for 3-keto-desogestrel at single dose is significantly lower than the mean AUC0 to 24 at steady state. This indicates that the kinetics of 3-keto-desogestrel are non-linear due to an increase in binding of 3-keto-desogestrel to sex hormone-binding globulin in the cycle, attributed to increased sex hormone-binding globulin levels which are induced by the daily administration of ethinyl estradiol. Sex hormone-binding globulin levels increased significantly in the third treatment cycle from day 1 (150 ± 64 nmol/L) to day 21 (230 ± 59 nmol/L).
- The elimination half-life for 3-keto-desogestrel is approximately 38 ± 20 hours at steady state. In addition to 3-keto-desogestrel, other phase I metabolites are 3α-OH-desogestrel, 3ß-OH-desogestrel, and 3α-OH-5α-H-desogestrel. These other metabolites are not known to have any pharmacologic effects, and are further converted in part by conjugation (phase II metabolism) into polar metabolites, mainly sulfates and glucuronides.
- Ethinyl estradiol is rapidly and almost completely absorbed. In the third cycle of use after a dose of Apri, the relative bioavailability is approximately 83%.
- In the third cycle of use after a single dose of Apri, maximum concentrations of ethinyl estradiol of 95 ± 34 pg/mL are reached at 1.5 ± 0.8 hours. The AUC0 to ∞ is 1,471 ± 268 pg/mL • hr after a single dose. At steady state, attained from at least day 19 onwards, maximum ethinyl estradiol concentrations of 141 ± 48 pg/mL are reached at about 1.4 ± 0.7 hours. The minimum serum levels of ethinyl estradiol at steady state are 24 ± 8.3 pg/mL. The AUC0 to 24, at steady state is 1,117 ± 302 pg/mL • hr. The mean AUC0 to ∞ for ethinyl estradiol following a single dose during treatment cycle 3 does not significantly differ from the mean AUC0 to 24 at steady state. This finding indicates linear kinetics for ethinyl estradiol.
- The elimination half-life is 26 ± 6.8 hours at steady state. Ethinyl estradiol is subject to a significant degree of presystemic conjugation (phase II metabolism). Ethinyl estradiol escaping gut wall conjugation undergoes phase I metabolism and hepatic conjugation (phase II metabolism). Major phase I metabolites are 2-OH-ethinyl estradiol and 2-methoxy-ethinyl estradiol. Sulfate and glucuronide conjugates of both ethinyl estradiol and phase I metabolites, which are excreted in bile, can undergo enterohepatic circulation.
Nonclinical Toxicology
There is limited information regarding Desogestrel and Ethinyl Estradiol Nonclinical Toxicology in the drug label.
Clinical Studies
There is limited information regarding Desogestrel and Ethinyl Estradiol Clinical Studies in the drug label.
How Supplied
- Desogestrel and ethinyl estradiol tablets 28 tablets blister cards contain 21 round, film-coated, unscored, biconvex, rose-colored tablets and 7 round, unscored white tablets. Each rose-colored tablet (debossed with “dp” on one side and “575” on the other side) contains 0.15 mg desogestrel and 0.03 mg ethinyl estradiol, USP. Each white tablet (debossed with “dp” on one side and “570” on the other side) contains inert ingredients.
- Cartons of 6 blister cards.
Storage
- Store at 20° to 25°C (68° to 77°F)
Images
Drug Images
{{#ask: Page Name::Desogestrel and Ethinyl Estradiol |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel

{{#ask: Label Page::Desogestrel and Ethinyl Estradiol |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
There is limited information regarding Desogestrel and Ethinyl Estradiol Patient Counseling Information in the drug label.
Precautions with Alcohol
- Alcohol-Desogestrel and Ethinyl Estradiol interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
Look-Alike Drug Names
There is limited information regarding Desogestrel and Ethinyl Estradiol Look-Alike Drug Names in the drug label.
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
{{#subobject:
|Label Page=Desogestrel and Ethinyl Estradiol |Label Name=Deso Package1c.png
}}
{{#subobject:
|Label Page=Desogestrel and Ethinyl Estradiol |Label Name=Desog Package2c.png
}}