Typhlitis: Difference between revisions
No edit summary |
No edit summary |
||
| (81 intermediate revisions by 2 users not shown) | |||
| Line 1: | Line 1: | ||
__NOTOC__ | __NOTOC__ | ||
{{SI}} | {{SI}} | ||
{{CMG}} | {{CMG}} {{AE}} {{SHM}} | ||
{{SK}} | {{SK}} Neutropenic colitis; Neutropenic enterocolitis; cecitis | ||
==Overview== | ==Overview== | ||
Typhlitis | Typhlitis is most commonly seen in [[Neutropenic fever|neutropenic]] [[patients]] receiving [[chemotherapy]] for a cancer. It is also been seen in people with [[aplastic anemia]], [[lymphoma]], [[Acquired immunodeficiency syndrome|acquired immunodeficiency]] [[syndrome]], as well as people who have had a [[Kidney transplant|kidney transplant]]. Typhlitis is distinguished by [[edema]] and [[inflammation]] of the [[cecum]], [[ascending colon]], and, in some cases, [[terminal ileum]]. Transmural [[necrosis]], [[perforation]], and [[mortality]] can occur as a result of the [[inflammation]]. The exact cause of the [[condition]] is unknown, but it is most likely caused by a combination of [[ischemia]], [[infection]] (particularly with [[cytomegalovirus]]), [[mucosal]] [[hemorrhage]], and possibly [[neoplastic]] [[Infiltration (medical)|infiltration]]. The treatment includes [[bowel]] rest, [[parenteral nutrition]], [[antibiotics]], and intensive fluid and [[electrolyte]] replacement. | ||
==Historical Perspective== | ==Historical Perspective== | ||
*In 1970, Wagner et al found and described typhlitis as [[necrotizing]] [[colitis]] after [[autopsy]] of 191 [[leukemic]] children with terminal illness at the Texas Children's Hospital, Baylor College of Medicine, Houston, between 1958 and 1970.<ref name="KatzMahoney1990">{{cite journal|last1=Katz|first1=Julie A.|last2=Mahoney|first2=Donald H.|last3=Fernbach|first3=Donald J.|last4=Wagner|first4=Milton L.|last5=Gresik|first5=Mary V.|title=Typhlitis. An 18-year experience and postmortem review|journal=Cancer|volume=65|issue=4|year=1990|pages=1041–1047|issn=0008-543X|doi=10.1002/1097-0142(19900215)65:4<1041::AID-CNCR2820650433>3.0.CO;2-A}}</ref> | |||
==Classification== | ==Classification== | ||
There is no established system for the | *There is no established system for the classification of Typhlitis. | ||
==Pathophysiology== | ==Pathophysiology== | ||
*The precise [[pathophysiology]] of [[Neutropenic sepsis|Neutropenic]] [[enterocolitis]] is unknown.<ref name="pmid10593241">{{cite journal| author=Urbach DR, Rotstein OD| title=Typhlitis. | journal=Can J Surg | year= 1999 | volume= 42 | issue= 6 | pages= 415-9 | pmid=10593241 | doi= | pmc=3795130 | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=10593241 }}</ref> | |||
*The primary variables in illness beginning appear to be [[intestinal]] [[mucosal]] injury, [[Neutropenia causes|neutropenia]], and the [[immunocompromised]] status of the [[patients]].<ref name="pmid19646645">{{cite journal| author=Cloutier RL| title=Neutropenic enterocolitis. | journal=Emerg Med Clin North Am | year= 2009 | volume= 27 | issue= 3 | pages= 415-22 | pmid=19646645 | doi=10.1016/j.emc.2009.04.002 | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=19646645 }}</ref> | |||
*[[Gram-negative]] rods, [[gram-positive cocci]], [[enterococci]], [[fungi]], and [[viruses]] have all been blamed for the outbreak.<ref name="pmid28104979">{{cite journal| author=Rodrigues FG, Dasilva G, Wexner SD| title=Neutropenic enterocolitis. | journal=World J Gastroenterol | year= 2017 | volume= 23 | issue= 1 | pages= 42-47 | pmid=28104979 | doi=10.3748/wjg.v23.i1.42 | pmc=5221285 | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=28104979 }}</ref><ref name="pmid31869058">{{cite journal| author=| title=StatPearls | journal= | year= 2021 | volume= | issue= | pages= | pmid=31869058 | doi= | pmc= | url= }}</ref> | |||
*These early circumstances cause [[intestinal]] [[edema]], engorged [[veins]], and a disrupted mucosal surface, making the mucosa more susceptible to [[bacterial]] intramural invasion. | |||
*The [[distension]] and [[necrosis]] generated by [[Chemotherapy agents|chemotherapy]] drugs directly influence [[intestinal]] motility. | |||
*Superimposed [[infections]] caused by [[bacteria]],[[fungi]] and [[viruses]] can also disrupts the already damaged [[mucosa]] leading further [[intestinal]] [[edema]], distension and [[necrosis]] of [[intestinal]] layer which lead to [[intestinal]] [[perforation]]. | |||
[ | |||
[ | |||
==Causes== | |||
===Causes by Organ System=== | ===Causes by Organ System=== | ||
{|style="width:80%; height:100px" border="1" | {| style="width:80%; height:100px" border="1" | ||
| | | style="width:25%" bgcolor="LightSteelBlue" ; border="1" |'''Cardiovascular''' | ||
| | | style="width:75%" bgcolor="Beige" ; border="1" |No underlying causes | ||
|- | |- | ||
|bgcolor="LightSteelBlue"| '''Chemical/Poisoning''' | | bgcolor="LightSteelBlue" |'''Chemical/Poisoning''' | ||
|bgcolor="Beige"| No underlying causes | | bgcolor="Beige" |No underlying causes | ||
|- | |- | ||
|-bgcolor="LightSteelBlue" | |- bgcolor="LightSteelBlue" | ||
| '''Dental''' | |'''Dental''' | ||
|bgcolor="Beige"| No underlying causes | | bgcolor="Beige" |No underlying causes | ||
|- | |- | ||
|-bgcolor="LightSteelBlue" | |- bgcolor="LightSteelBlue" | ||
| '''Dermatologic''' | |'''Dermatologic''' | ||
|bgcolor="Beige"| No underlying causes | | bgcolor="Beige" |No underlying causes | ||
|- | |- | ||
|-bgcolor="LightSteelBlue" | |- bgcolor="LightSteelBlue" | ||
| '''Drug Side Effect''' | |'''Drug Side Effect''' | ||
|bgcolor="Beige"| [[Doxorubicin Hydrochloride]], [[ | | bgcolor="Beige" |[[Doxorubicin Hydrochloride]], [[cytosine arabinoside]], [[gemcitabine]], [[vincristine]], [[doxorubicin]], [[cyclophosphamide]], [[5-fluorouracil]], [[leucovorin]], and [[daunorubicin]] are some of the drugs used to treat cancer.<ref name="pmid281049792">{{cite journal| author=Rodrigues FG, Dasilva G, Wexner SD| title=Neutropenic enterocolitis. | journal=World J Gastroenterol | year= 2017 | volume= 23 | issue= 1 | pages= 42-47 | pmid=28104979 | doi=10.3748/wjg.v23.i1.42 | pmc=5221285 | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=28104979 }}</ref>[[Antibiotics]], [[sulfasalazine]], and [[immunosuppressive]] medication for [[organ transplantation]]<ref name="ChakravartyScott1992">{{cite journal|last1=Chakravarty|first1=K.|last2=Scott|first2=D. G. I.|last3=Mccann|first3=B. G.|title=FATAL NEUTROPENIC ENTEROCOLITIS ASSOCIATED WITH SULPHASALAZINE THERAPY FOR RHEUMATOID ARTHRITIS|journal=Rheumatology|volume=31|issue=5|year=1992|pages=351–353|issn=1462-0324|doi=10.1093/rheumatology/31.5.351}}</ref><ref name="pmid11038099">{{cite journal| author=Bibbo C, Barbieri RA, Deitch EA, Brolin RE| title=Neutropenic enterocolitis in a trauma patient during antibiotic therapy for osteomyelitis. | journal=J Trauma | year= 2000 | volume= 49 | issue= 4 | pages= 760-3 | pmid=11038099 | doi=10.1097/00005373-200010000-00029 | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=11038099 }}</ref> | ||
|- | |- | ||
|-bgcolor="LightSteelBlue" | |- bgcolor="LightSteelBlue" | ||
| '''Ear Nose Throat''' | |'''Ear Nose Throat''' | ||
|bgcolor="Beige"| No underlying causes | | bgcolor="Beige" |No underlying causes | ||
|- | |- | ||
|-bgcolor="LightSteelBlue" | |- bgcolor="LightSteelBlue" | ||
| '''Endocrine''' | |'''Endocrine''' | ||
|bgcolor="Beige"| No underlying causes | | bgcolor="Beige" |No underlying causes | ||
|- | |- | ||
|-bgcolor="LightSteelBlue" | |- bgcolor="LightSteelBlue" | ||
| '''Environmental''' | |'''Environmental''' | ||
|bgcolor="Beige"| No underlying causes | | bgcolor="Beige" |No underlying causes | ||
|- | |- | ||
|-bgcolor="LightSteelBlue" | |- bgcolor="LightSteelBlue" | ||
| '''Gastroenterologic''' | |'''Gastroenterologic''' | ||
|bgcolor="Beige"| No underlying causes | | bgcolor="Beige" |No underlying causes | ||
|- | |- | ||
|-bgcolor="LightSteelBlue" | |- bgcolor="LightSteelBlue" | ||
| '''Genetic''' | |'''Genetic''' | ||
|bgcolor="Beige"| No underlying causes | | bgcolor="Beige" |No underlying causes | ||
|- | |- | ||
|-bgcolor="LightSteelBlue" | |- bgcolor="LightSteelBlue" | ||
| '''Hematologic''' | |'''Hematologic''' | ||
|bgcolor="Beige"| | | bgcolor="Beige" |Adults with [[Hematologic malignancy|hematologic]] malignancies such [[leukemia]], [[lymphoma]], [[multiple myeloma]], [[aplastic anemia]], and [[Myelodysplastic syndrome|myelodysplastic syndromes]].<ref name="pmid16319675">{{cite journal| author=Davila ML| title=Neutropenic enterocolitis. | journal=Curr Opin Gastroenterol | year= 2006 | volume= 22 | issue= 1 | pages= 44-7 | pmid=16319675 | doi=10.1097/01.mog.0000198073.14169.3b | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=16319675 }}</ref> | ||
|- | |- | ||
|-bgcolor="LightSteelBlue" | |- bgcolor="LightSteelBlue" | ||
| '''Iatrogenic''' | |'''Iatrogenic''' | ||
|bgcolor="Beige"| No underlying causes | | bgcolor="Beige" |No underlying causes | ||
|- | |- | ||
|-bgcolor="LightSteelBlue" | |- bgcolor="LightSteelBlue" | ||
| '''Infectious Disease''' | |'''Infectious Disease''' | ||
|bgcolor="Beige"| No underlying causes | | bgcolor="Beige" |No underlying causes | ||
|- | |- | ||
|-bgcolor="LightSteelBlue" | |- bgcolor="LightSteelBlue" | ||
| '''Musculoskeletal/Orthopedic''' | |'''Musculoskeletal/Orthopedic''' | ||
|bgcolor="Beige"| No underlying causes | | bgcolor="Beige" |No underlying causes | ||
|- | |- | ||
|-bgcolor="LightSteelBlue" | |- bgcolor="LightSteelBlue" | ||
| '''Neurologic''' | |'''Neurologic''' | ||
|bgcolor="Beige"| No underlying causes | | bgcolor="Beige" |No underlying causes | ||
|- | |- | ||
|-bgcolor="LightSteelBlue" | |- bgcolor="LightSteelBlue" | ||
| '''Nutritional/Metabolic''' | |'''Nutritional/Metabolic''' | ||
|bgcolor="Beige"| No underlying causes | | bgcolor="Beige" |No underlying causes | ||
|- | |- | ||
|-bgcolor="LightSteelBlue" | |- bgcolor="LightSteelBlue" | ||
| '''Obstetric/Gynecologic''' | |'''Obstetric/Gynecologic''' | ||
|bgcolor="Beige"| No underlying causes | | bgcolor="Beige" |No underlying causes | ||
|- | |- | ||
|-bgcolor="LightSteelBlue" | |- bgcolor="LightSteelBlue" | ||
| '''Oncologic''' | |'''Oncologic''' | ||
|bgcolor="Beige"| No underlying causes | | bgcolor="Beige" |No underlying causes | ||
|- | |- | ||
|-bgcolor="LightSteelBlue" | |- bgcolor="LightSteelBlue" | ||
| '''Ophthalmologic''' | |'''Ophthalmologic''' | ||
|bgcolor="Beige"| No underlying causes | | bgcolor="Beige" |No underlying causes | ||
|- | |- | ||
|-bgcolor="LightSteelBlue" | |- bgcolor="LightSteelBlue" | ||
| '''Overdose/Toxicity''' | |'''Overdose/Toxicity''' | ||
|bgcolor="Beige"| No underlying causes | | bgcolor="Beige" |No underlying causes | ||
|- | |- | ||
|-bgcolor="LightSteelBlue" | |- bgcolor="LightSteelBlue" | ||
| '''Psychiatric''' | |'''Psychiatric''' | ||
|bgcolor="Beige"| No underlying causes | | bgcolor="Beige" |No underlying causes | ||
|- | |- | ||
|-bgcolor="LightSteelBlue" | |- bgcolor="LightSteelBlue" | ||
| '''Pulmonary''' | |'''Pulmonary''' | ||
|bgcolor="Beige"| No underlying causes | | bgcolor="Beige" |No underlying causes | ||
|- | |- | ||
|-bgcolor="LightSteelBlue" | |- bgcolor="LightSteelBlue" | ||
| '''Renal/Electrolyte''' | |'''Renal/Electrolyte''' | ||
|bgcolor="Beige"| No underlying causes | | bgcolor="Beige" |No underlying causes | ||
|- | |- | ||
|-bgcolor="LightSteelBlue" | |- bgcolor="LightSteelBlue" | ||
| '''Rheumatology/Immunology/Allergy''' | |'''Rheumatology/Immunology/Allergy''' | ||
|bgcolor="Beige"| No underlying causes | | bgcolor="Beige" |No underlying causes | ||
|- | |- | ||
|-bgcolor="LightSteelBlue" | |- bgcolor="LightSteelBlue" | ||
| '''Sexual''' | |'''Sexual''' | ||
|bgcolor="Beige"| No underlying causes | | bgcolor="Beige" |No underlying causes | ||
|- | |- | ||
|-bgcolor="LightSteelBlue" | |- bgcolor="LightSteelBlue" | ||
| '''Trauma''' | |'''Trauma''' | ||
|bgcolor="Beige"| No underlying causes | | bgcolor="Beige" |No underlying causes | ||
|- | |- | ||
|-bgcolor="LightSteelBlue" | |- bgcolor="LightSteelBlue" | ||
| '''Urologic''' | |'''Urologic''' | ||
|bgcolor="Beige"| No underlying causes | | bgcolor="Beige" |No underlying causes | ||
|- | |- | ||
|-bgcolor="LightSteelBlue" | |- bgcolor="LightSteelBlue" | ||
| '''Miscellaneous''' | |'''Miscellaneous''' | ||
|bgcolor="Beige"| No underlying causes | | bgcolor="Beige" |No underlying causes | ||
|- | |- | ||
|} | |} | ||
{{columns-list}} | |||
{{columns-list | |||
}} | |||
==Differentiating | ==Differentiating Typhlitis from other Diseases== | ||
Typhlitis must be distinguished from other diseases that exhibit [[symptoms]] such as [[Feve|fever]], [[abdomrinal pain|abdominal pain]], and [[diarrhea]].<ref name="pmid16632437">{{cite journal| author=Cardona Zorrilla AF, Reveiz Herault L, Casasbuenas A, Aponte DM, Ramos PL| title=Systematic review of case reports concerning adults suffering from neutropenic enterocolitis. | journal=Clin Transl Oncol | year= 2006 | volume= 8 | issue= 1 | pages= 31-8 | pmid=16632437 | doi=10.1007/s12094-006-0092-y | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=16632437 }}</ref> | |||
*[[Clostridium difficile|1. Clostridium difficile]] [[infection]]<ref name="pmid30945014">{{cite journal| author=Czepiel J, Dróżdż M, Pituch H, Kuijper EJ, Perucki W, Mielimonka A | display-authors=etal| title=Clostridium difficile infection: review. | journal=Eur J Clin Microbiol Infect Dis | year= 2019 | volume= 38 | issue= 7 | pages= 1211-1221 | pmid=30945014 | doi=10.1007/s10096-019-03539-6 | pmc=6570665 | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=30945014 }}</ref> | |||
*[[Cytomegalovirus|2. Cytomegalovirus]] [[colitis]]<ref name="pmid26877608">{{cite journal| author=Pillet S, Pozzetto B, Roblin X| title=Cytomegalovirus and ulcerative colitis: Place of antiviral therapy. | journal=World J Gastroenterol | year= 2016 | volume= 22 | issue= 6 | pages= 2030-45 | pmid=26877608 | doi=10.3748/wjg.v22.i6.2030 | pmc=4726676 | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=26877608 }}</ref> | |||
[ | *[[Norovirus|3. Norovirus]] [[infection]]<ref name="pmid31335045">{{cite journal| author=| title=StatPearls | journal= | year= 2021 | volume= | issue= | pages= | pmid=31335045 | doi= | pmc= | url= }}</ref> | ||
*[[Graft versus host disease|4. Graft versus host disease]]<ref name="pmid31466596">{{cite journal| author=Ramachandran V, Kolli SS, Strowd LC| title=Review of Graft-Versus-Host Disease. | journal=Dermatol Clin | year= 2019 | volume= 37 | issue= 4 | pages= 569-582 | pmid=31466596 | doi=10.1016/j.det.2019.05.014 | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=31466596 }}</ref> | |||
*[[Acute appendicitis|5. Acute appendicitis]]<ref name="pmid26460662">{{cite journal| author=Bhangu A, Søreide K, Di Saverio S, Assarsson JH, Drake FT| title=Acute appendicitis: modern understanding of pathogenesis, diagnosis, and management. | journal=Lancet | year= 2015 | volume= 386 | issue= 10000 | pages= 1278-1287 | pmid=26460662 | doi=10.1016/S0140-6736(15)00275-5 | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=26460662 }}</ref> | |||
*6. [[Ischemic colitis]]<ref name="pmid19109863">{{cite journal| author=Theodoropoulou A, Koutroubakis IE| title=Ischemic colitis: clinical practice in diagnosis and treatment. | journal=World J Gastroenterol | year= 2008 | volume= 14 | issue= 48 | pages= 7302-8 | pmid=19109863 | doi=10.3748/wjg.14.7302 | pmc=2778113 | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=19109863 }}</ref> | |||
* | |||
==Epidemiology and Demographics== | ==Epidemiology and Demographics== | ||
*The prevalence of [[Febrile neutropenia|Neutropenic]] [[enterocolitis]] varies between studies. Gorschlüter et al. conducted a systematic review and found that the incidence rate from 21 studies was 5.3 percent in patients hospitalized for [[hematological malignancies]], high-dose [[chemotherapy]] for [[solid tumors]], or [[aplastic anemia]]. Another [[cohort study]] discovered it in 3.5% of 317 severely [[neutropenic]] patients. The [[prevalence]] of [[neutropenic]] [[enterocolitis]] has been increasing in tandem with the increased use of [[chemotherapy]], especially the agents known for causing [[mucositis]].<ref name="pmid15946304">{{cite journal| author=Gorschlüter M, Mey U, Strehl J, Ziske C, Schepke M, Schmidt-Wolf IG | display-authors=etal| title=Neutropenic enterocolitis in adults: systematic analysis of evidence quality. | journal=Eur J Haematol | year= 2005 | volume= 75 | issue= 1 | pages= 1-13 | pmid=15946304 | doi=10.1111/j.1600-0609.2005.00442.x | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=15946304 }}</ref><ref name="pmid17023562">{{cite journal| author=Aksoy DY, Tanriover MD, Uzun O, Zarakolu P, Ercis S, Ergüven S | display-authors=etal| title=Diarrhea in neutropenic patients: a prospective cohort study with emphasis on neutropenic enterocolitis. | journal=Ann Oncol | year= 2007 | volume= 18 | issue= 1 | pages= 183-189 | pmid=17023562 | doi=10.1093/annonc/mdl337 | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=17023562 }}</ref> | |||
*Patients with [[Hematologic malignancy|hematologic malignancie]][[Malignancies|s]] are more likely to develop [[Febrile neutropenia|Neutropenic]] [[enterocolitis]] as a result of their underlying [[malignancy]] as well as their treatment regimens. [[Febrile neutropenia|Neutropenic]] [[enterocolitis]] has also been reported in patients taking [[immunosuppressive]] [[medications]], patients diagnosed with solid [[tumors]] and [[autoimmune]] conditions.<ref name="pmid23196957">{{cite journal| author=Nesher L, Rolston KV| title=Neutropenic enterocolitis, a growing concern in the era of widespread use of aggressive chemotherapy. | journal=Clin Infect Dis | year= 2013 | volume= 56 | issue= 5 | pages= 711-7 | pmid=23196957 | doi=10.1093/cid/cis998 | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=23196957 }}</ref> | |||
[ | |||
[ | |||
[ | |||
[ | |||
==Risk Factors== | ==Risk Factors== | ||
Common risk factors in the development of Typhlitis include [[hematological]], solid [[tumors]], [[Neutropenic fever|neutropenic]] and [[Immunocompromised]] individuals.<ref name="BiasoliNucci19972">{{cite journal|last1=Biasoli|first1=I|last2=Nucci|first2=M|last3=Spector|first3=N|last4=Portugal|first4=R|last5=Domingues|first5=A|last6=Pulcheri|first6=W|title=Risk factors for typhlitis|journal=Oncology Reports|year=1997|issn=1021-335X|doi=10.3892/or.4.5.1029}}</ref> | |||
==Screening== | ==Screening== | ||
There is insufficient evidence to recommend routine | There is insufficient evidence to recommend routine [[screening]] for [[Febrile neutropenia|Neutropenic]] [[enterocolitis]]. | ||
==Natural History, Complications, and Prognosis== | ==Natural History, Complications, and Prognosis== | ||
*Common complications of [[Febrile neutropenia|Neutropenic]] [[enterocolitis]] include [[perforation]], [[peritonitis]], [[sepsis]], and [[abscess]] formation, which are all caused by the [[pathology]] ([[bowel]] wall [[inflammation]]). Other risks are related to [[pancytopenia]] include [[thrombocytopenia]]-related extreme bleeding and delayed healing.<ref name="pmid159463042">{{cite journal| author=Gorschlüter M, Mey U, Strehl J, Ziske C, Schepke M, Schmidt-Wolf IG | display-authors=etal| title=Neutropenic enterocolitis in adults: systematic analysis of evidence quality. | journal=Eur J Haematol | year= 2005 | volume= 75 | issue= 1 | pages= 1-13 | pmid=15946304 | doi=10.1111/j.1600-0609.2005.00442.x | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=15946304 }} | |||
*[[Prognosis]] is generally poor, and the [[mortality rate]] of patients with [[Febrile neutropenia|Neutropenic]] [[enterocolitis]] is as high as 50% especially in patients with transmural [[inflammation]] or [[intestinal perforation]]</ref><ref name="pmid1727660">{{cite journal| author=Wade DS, Nava HR, Douglass HO| title=Neutropenic enterocolitis. Clinical diagnosis and treatment. | journal=Cancer | year= 1992 | volume= 69 | issue= 1 | pages= 17-23 | pmid=1727660 | doi=10.1002/1097-0142(19920101)69:1<17::aid-cncr2820690106>3.0.co;2-x | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=1727660 }}</ref> | |||
*Neutrophilic enterocolitis has documented mortality rates as high as 50%, especially in patients with transmural inflammation or intestinal rupture. | |||
==Diagnosis== | |||
[[Febrile neutropenia|Neutropenic]] [[enterocolitis]] is typically diagnosed based on a combination of [[clinical]] and [[Radiology|radiological]] findings.<ref name="pmid8218694">{{cite journal| author=Sloas MM, Flynn PM, Kaste SC, Patrick CC| title=Typhlitis in children with cancer: a 30-year experience. | journal=Clin Infect Dis | year= 1993 | volume= 17 | issue= 3 | pages= 484-90 | pmid=8218694 | doi=10.1093/clinids/17.3.484 | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=8218694 }}</ref> | |||
===Diagnostic Study of Choice=== | ===Diagnostic Study of Choice=== | ||
There are no established criteria for the diagnosis of typhlitis. | |||
There are no established criteria for the diagnosis of | |||
===History and Symptoms=== | ===History and Symptoms=== | ||
The | The most common symptoms of typhlitis include [[fever]], [[abdominal pain]], and [[diarrhea]]. In severe cases, [[diarrhea]] can be [[Bloody diarrhea|bloody]]. [[Abdominal distension]] and [[paralytic ileus]] may also occur in patients.<ref name="pmid231969572">{{cite journal| author=Nesher L, Rolston KV| title=Neutropenic enterocolitis, a growing concern in the era of widespread use of aggressive chemotherapy. | journal=Clin Infect Dis | year= 2013 | volume= 56 | issue= 5 | pages= 711-7 | pmid=23196957 | doi=10.1093/cid/cis998 | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=23196957 }}</ref> | ||
===Physical Examination=== | ===Physical Examination=== | ||
Common physical examination of patients with [[Febrile neutropenia|Neutropenic]] [[enterocolitis]] is usually remarkable for [[Abdominal]] discomfort which can be [[diffuse]] or [[Localized disease|localized]], with the [[Right lower quadrant abdominal pain resident survival guide|right lower quadrant]] being the most common location. A rigid abdomen could be an indication of [[bowel perforation]].<ref name="NesherRolston20122">{{cite journal|last1=Nesher|first1=L.|last2=Rolston|first2=K. V. I.|title=Neutropenic Enterocolitis, a Growing Concern in the Era of Widespread Use of Aggressive Chemotherapy|journal=Clinical Infectious Diseases|volume=56|issue=5|year=2012|pages=711–717|issn=1058-4838|doi=10.1093/cid/cis998}}</ref> | |||
===Laboratory Findings=== | ===Laboratory Findings=== | ||
Laboratory findings consistent with the diagnosis of typhlitis include [[Neutropenia causes|neutropenia]] with absolute neutrophil count <500 cells/microL, [[thrombocytopenia]] ranged from 4000/pl to 120,000/pl.<ref name="pmid2404562">{{cite journal| author=Katz JA, Wagner ML, Gresik MV, Mahoney DH, Fernbach DJ| title=Typhlitis. An 18-year experience and postmortem review. | journal=Cancer | year= 1990 | volume= 65 | issue= 4 | pages= 1041-7 | pmid=2404562 | doi=10.1002/1097-0142(19900215)65:4<1041::aid-cncr2820650433>3.0.co;2-a | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=2404562 }}</ref> | |||
Laboratory findings consistent with the diagnosis of [ | |||
[ | |||
=== | |||
===Ultrasound=== | |||
*[[Ultrasound]] (US) may be helpful in the [[diagnosis]] of [[Febrile neutropenia|Neutropenic]] [[enterocolitis]]. Findings on an [[ultrasound]] suggestive of [[Febrile neutropenia|Neutropenic]] [[enterocolitis]] include circumferential wall thickening and prominent [[submucosa]] .<ref name="TamburriniSetola2018">{{cite journal|last1=Tamburrini|first1=Stefania|last2=Setola|first2=Francesca Rosa|last3=Belfiore|first3=Maria Paola|last4=Saturnino|first4=Pietro Paolo|last5=Della Casa|first5=Maria Gabriella|last6=Sarti|first6=Giuseppe|last7=Abete|first7=Roberta|last8=Marano|first8=Ines|title=Ultrasound diagnosis of typhlitis|journal=Journal of Ultrasound|volume=22|issue=1|year=2018|pages=103–106|issn=1876-7931|doi=10.1007/s40477-018-0333-2}}</ref> | |||
===X-ray=== | ===X-ray=== | ||
An [[x-ray]] may be helpful in the [[diagnosis]] of Typhlitis but nonspecific. Findings on an [[x-ray]] suggestive of [[Febrile neutropenia|Neutropenic]] [[enterocolitis]] include inflated [[cecum]] with dilated [[small bowel]] loops, can detect free air.<ref name="pmid727660">{{cite journal| author=Terzis JK, Daniel RK, Williams HB, Spencer PS| title=Benign fatty tumors of the peripheral nerves. | journal=Ann Plast Surg | year= 1978 | volume= 1 | issue= 2 | pages= 193-216 | pmid=727660 | doi=10.1097/00000637-197803000-00011 | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=727660 }}</ref> | |||
=== | |||
===CT Scan=== | |||
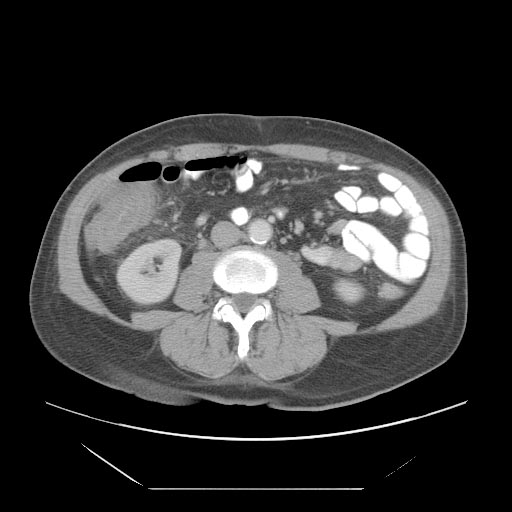
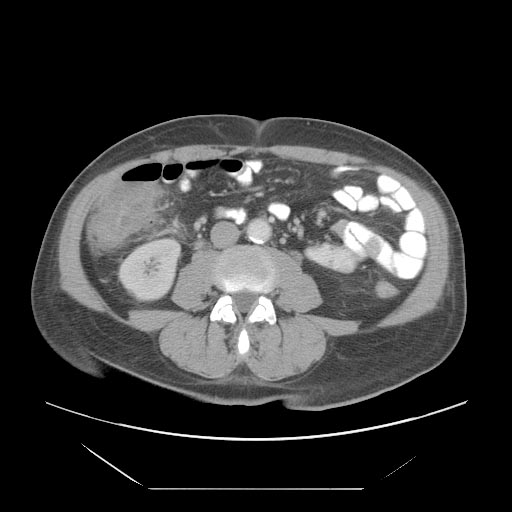
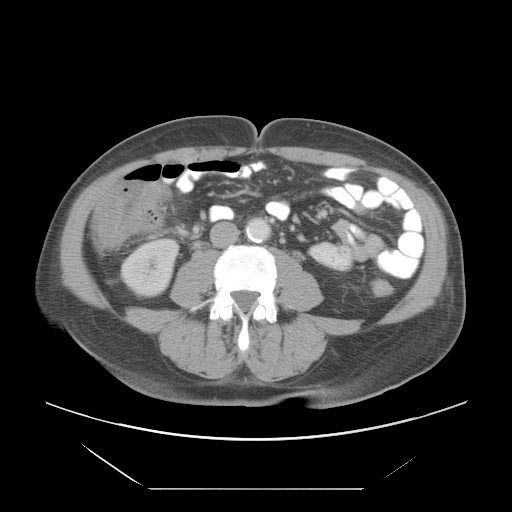
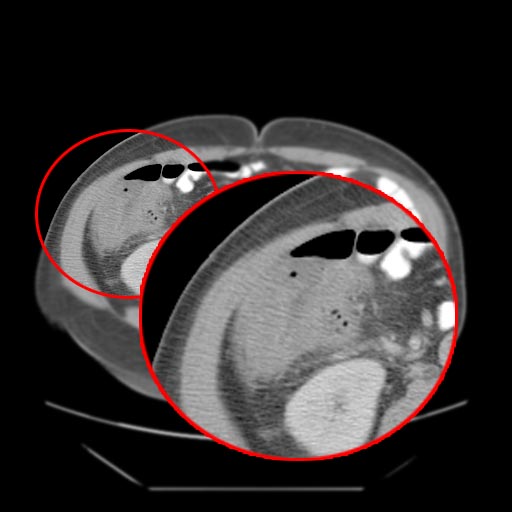
*A [[computed tomography]] (CT) scan of the [[abdomen]] may be helpful in the diagnosis of [[Febrile neutropenia|Neutropenic]] [[enterocolitis]]. Findings on CT scan suggestive of [[Febrile neutropenia|Neutropenic]] [[enterocolitis]] include [[Intestinal wall]] thickening, [[mesenteric|mesenteric]] stranding, [[intestinal]] [[dilatation]], [[Pneumatosis intestinalis CT|pneumatosis, ]]distention and circumferential thickening of the cecal wall.<ref name="KirkpatrickGreenberg2003">{{cite journal|last1=Kirkpatrick|first1=Iain D. C.|last2=Greenberg|first2=Howard M.|title=Gastrointestinal Complications in the Neutropenic Patient: Characterization and Differentiation with Abdominal CT|journal=Radiology|volume=226|issue=3|year=2003|pages=668–674|issn=0033-8419|doi=10.1148/radiol.2263011932}}</ref> | |||
<gallery> | <gallery> | ||
File:Typhlitis-001.jpg | |||
File:Typhlitis-002.jpg | |||
Typhlitis-001.jpg | File:Typhlitis-003.jpg | ||
File:Typhlitis-004.jpg | |||
Typhlitis-002.jpg | |||
Typhlitis-003.jpg | |||
Typhlitis-004.jpg | |||
</gallery> | </gallery> | ||
=== | ==Treatment== | ||
===Medical Therapy=== | ===Medical Therapy=== | ||
*The mainstay of treatment for [[Febrile neutropenia|Neutropenic]] [[enterocolitis]] consists of both supportive [[therapy]] and [[antimicrobials]]<ref name="pmid19231539">{{cite journal| author=Mullassery D, Bader A, Battersby AJ, Mohammad Z, Jones EL, Parmar C | display-authors=etal| title=Diagnosis, incidence, and outcomes of suspected typhlitis in oncology patients--experience in a tertiary pediatric surgical center in the United Kingdom. | journal=J Pediatr Surg | year= 2009 | volume= 44 | issue= 2 | pages= 381-5 | pmid=19231539 | doi=10.1016/j.jpedsurg.2008.10.094 | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=19231539 }}</ref> | |||
*Supportive [[therapy]] for [[Febrile neutropenia|Neutropenic]] [[colitis]] include [[bowel]] rest with [[Nasogastric aspiration|nasogastric]] suction, [[intravenous fluids]], and, if necessary, [[Parenteral nutrition|parenteral nutrition.]] | |||
* | *[[Empiric therapy]] for [[Febrile neutropenia|Neutropenic]] [[colitis]] depends on [[antimicrobial]] exposure, [[bacteremia]] and local resistance pattern. | ||
*Pipericillin-tazobactum, carbapenam, or an antipseudomonal [[cephalosporin]] is recommended among patients who develop [[neutropenic]] [[colitis]] and [[vancomycin]] is considered in case of [[mucositis]] is suspected, which is against [[gram positive bacteria]].<ref name="pmid21258094">{{cite journal| author=Freifeld AG, Bow EJ, Sepkowitz KA, Boeckh MJ, Ito JI, Mullen CA | display-authors=etal| title=Clinical practice guideline for the use of antimicrobial agents in neutropenic patients with cancer: 2010 update by the infectious diseases society of america. | journal=Clin Infect Dis | year= 2011 | volume= 52 | issue= 4 | pages= e56-93 | pmid=21258094 | doi=10.1093/cid/cir073 | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=21258094 }}</ref><ref name="pmid10037047">{{cite journal| author=Salazar R, Solá C, Maroto P, Tabernero JM, Brunet J, Verger G | display-authors=etal| title=Infectious complications in 126 patients treated with high-dose chemotherapy and autologous peripheral blood stem cell transplantation. | journal=Bone Marrow Transplant | year= 1999 | volume= 23 | issue= 1 | pages= 27-33 | pmid=10037047 | doi=10.1038/sj.bmt.1701520 | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=10037047 }}</ref> | |||
: | |||
===Surgery=== | |||
*All individuals with [[Febrile neutropenia|Neutropenic]] [[enterocolitis]] should seek surgical advice as soon as possible.<ref name="pmid3484659">{{cite journal| author=Shamberger RC, Weinstein HJ, Delorey MJ, Levey RH| title=The medical and surgical management of typhlitis in children with acute nonlymphocytic (myelogenous) leukemia. | journal=Cancer | year= 1986 | volume= 57 | issue= 3 | pages= 603-9 | pmid=3484659 | doi=10.1002/1097-0142(19860201)57:3<603::aid-cncr2820570335>3.0.co;2-k | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=3484659 }}</ref> | |||
*[[Surgery]] is usually reserved for patients with either [[bowel perforation]], [[pneumoperitoneum]], or persistent [[gastrointestinal bleeding]]. | |||
:: | |||
===Primary Prevention=== | |||
Effective measures for the [[primary prevention]] of [[Febrile neutropenia|Neutropenic]] [[enterocolitis]] include early detection and treatment can help to avoid problems and improve outcomes in patients who have undergone intensive chemotherapy or a stem cell transplant.Treatment with G-CSF not only speeds recovery from neutropenia episodes that occur during chemotherapy, but it also reduces the risk of consequences including mucositis.<ref name="pmid7687319">{{cite journal| author=Steward WP| title=Granulocyte and granulocyte-macrophage colony-stimulating factors. | journal=Lancet | year= 1993 | volume= 342 | issue= 8864 | pages= 153-7 | pmid=7687319 | doi=10.1016/0140-6736(93)91350-u | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=7687319 }}</ref> | |||
===Secondary Prevention=== | |||
Effective measures for the [[secondary prevention]] include early surgical evaluation in the management of this condition, as it can be life-saving for some patients who present with a complicated [[Neutropenic fever|Neutropenic]] [[enterocolitis]]<ref name="VarkiArmitage1979">{{cite journal|last1=Varki|first1=Ajit P.|last2=Armitage|first2=James O.|last3=Feagler|first3=John R.|title=Typhlitis in acute leukemia.Successful treatment by early surgical intervention|journal=Cancer|volume=43|issue=2|year=1979|pages=695–697|issn=0008-543X|doi=10.1002/1097-0142(197902)43:2<695::AID-CNCR2820430242>3.0.CO;2-9}}</ref> | |||
: | |||
==References== | ==References== | ||
{{reflist|2}} | {{reflist|2}} | ||
Latest revision as of 00:54, 28 July 2021
|
WikiDoc Resources for Typhlitis |
|
Articles |
|---|
|
Most recent articles on Typhlitis |
|
Media |
|
Evidence Based Medicine |
|
Clinical Trials |
|
Ongoing Trials on Typhlitis at Clinical Trials.gov Clinical Trials on Typhlitis at Google
|
|
Guidelines / Policies / Govt |
|
US National Guidelines Clearinghouse on Typhlitis
|
|
Books |
|
News |
|
Commentary |
|
Definitions |
|
Patient Resources / Community |
|
Patient resources on Typhlitis Discussion groups on Typhlitis Directions to Hospitals Treating Typhlitis Risk calculators and risk factors for Typhlitis
|
|
Healthcare Provider Resources |
|
Causes & Risk Factors for Typhlitis |
|
Continuing Medical Education (CME) |
|
International |
|
|
|
Business |
|
Experimental / Informatics |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1] Associate Editor(s)-in-Chief: Shameera Shaik Masthan MBBS, DLO, DNB[2]
Synonyms and keywords: Neutropenic colitis; Neutropenic enterocolitis; cecitis
Overview
Typhlitis is most commonly seen in neutropenic patients receiving chemotherapy for a cancer. It is also been seen in people with aplastic anemia, lymphoma, acquired immunodeficiency syndrome, as well as people who have had a kidney transplant. Typhlitis is distinguished by edema and inflammation of the cecum, ascending colon, and, in some cases, terminal ileum. Transmural necrosis, perforation, and mortality can occur as a result of the inflammation. The exact cause of the condition is unknown, but it is most likely caused by a combination of ischemia, infection (particularly with cytomegalovirus), mucosal hemorrhage, and possibly neoplastic infiltration. The treatment includes bowel rest, parenteral nutrition, antibiotics, and intensive fluid and electrolyte replacement.
Historical Perspective
- In 1970, Wagner et al found and described typhlitis as necrotizing colitis after autopsy of 191 leukemic children with terminal illness at the Texas Children's Hospital, Baylor College of Medicine, Houston, between 1958 and 1970.[1]
Classification
- There is no established system for the classification of Typhlitis.
Pathophysiology
- The precise pathophysiology of Neutropenic enterocolitis is unknown.[2]
- The primary variables in illness beginning appear to be intestinal mucosal injury, neutropenia, and the immunocompromised status of the patients.[3]
- Gram-negative rods, gram-positive cocci, enterococci, fungi, and viruses have all been blamed for the outbreak.[4][5]
- These early circumstances cause intestinal edema, engorged veins, and a disrupted mucosal surface, making the mucosa more susceptible to bacterial intramural invasion.
- The distension and necrosis generated by chemotherapy drugs directly influence intestinal motility.
- Superimposed infections caused by bacteria,fungi and viruses can also disrupts the already damaged mucosa leading further intestinal edema, distension and necrosis of intestinal layer which lead to intestinal perforation.
Causes
Causes by Organ System
| Cardiovascular | No underlying causes |
| Chemical/Poisoning | No underlying causes |
| Dental | No underlying causes |
| Dermatologic | No underlying causes |
| Drug Side Effect | Doxorubicin Hydrochloride, cytosine arabinoside, gemcitabine, vincristine, doxorubicin, cyclophosphamide, 5-fluorouracil, leucovorin, and daunorubicin are some of the drugs used to treat cancer.[6]Antibiotics, sulfasalazine, and immunosuppressive medication for organ transplantation[7][8] |
| Ear Nose Throat | No underlying causes |
| Endocrine | No underlying causes |
| Environmental | No underlying causes |
| Gastroenterologic | No underlying causes |
| Genetic | No underlying causes |
| Hematologic | Adults with hematologic malignancies such leukemia, lymphoma, multiple myeloma, aplastic anemia, and myelodysplastic syndromes.[9] |
| Iatrogenic | No underlying causes |
| Infectious Disease | No underlying causes |
| Musculoskeletal/Orthopedic | No underlying causes |
| Neurologic | No underlying causes |
| Nutritional/Metabolic | No underlying causes |
| Obstetric/Gynecologic | No underlying causes |
| Oncologic | No underlying causes |
| Ophthalmologic | No underlying causes |
| Overdose/Toxicity | No underlying causes |
| Psychiatric | No underlying causes |
| Pulmonary | No underlying causes |
| Renal/Electrolyte | No underlying causes |
| Rheumatology/Immunology/Allergy | No underlying causes |
| Sexual | No underlying causes |
| Trauma | No underlying causes |
| Urologic | No underlying causes |
| Miscellaneous | No underlying causes |
Differentiating Typhlitis from other Diseases
Typhlitis must be distinguished from other diseases that exhibit symptoms such as fever, abdominal pain, and diarrhea.[10]
- 1. Clostridium difficile infection[11]
- 2. Cytomegalovirus colitis[12]
- 3. Norovirus infection[13]
- 4. Graft versus host disease[14]
- 5. Acute appendicitis[15]
- 6. Ischemic colitis[16]
Epidemiology and Demographics
- The prevalence of Neutropenic enterocolitis varies between studies. Gorschlüter et al. conducted a systematic review and found that the incidence rate from 21 studies was 5.3 percent in patients hospitalized for hematological malignancies, high-dose chemotherapy for solid tumors, or aplastic anemia. Another cohort study discovered it in 3.5% of 317 severely neutropenic patients. The prevalence of neutropenic enterocolitis has been increasing in tandem with the increased use of chemotherapy, especially the agents known for causing mucositis.[17][18]
- Patients with hematologic malignancies are more likely to develop Neutropenic enterocolitis as a result of their underlying malignancy as well as their treatment regimens. Neutropenic enterocolitis has also been reported in patients taking immunosuppressive medications, patients diagnosed with solid tumors and autoimmune conditions.[19]
Risk Factors
Common risk factors in the development of Typhlitis include hematological, solid tumors, neutropenic and Immunocompromised individuals.[20]
Screening
There is insufficient evidence to recommend routine screening for Neutropenic enterocolitis.
Natural History, Complications, and Prognosis
- Common complications of Neutropenic enterocolitis include perforation, peritonitis, sepsis, and abscess formation, which are all caused by the pathology (bowel wall inflammation). Other risks are related to pancytopenia include thrombocytopenia-related extreme bleeding and delayed healing.[21][22]
- Neutrophilic enterocolitis has documented mortality rates as high as 50%, especially in patients with transmural inflammation or intestinal rupture.
Diagnosis
Neutropenic enterocolitis is typically diagnosed based on a combination of clinical and radiological findings.[23]
Diagnostic Study of Choice
There are no established criteria for the diagnosis of typhlitis.
History and Symptoms
The most common symptoms of typhlitis include fever, abdominal pain, and diarrhea. In severe cases, diarrhea can be bloody. Abdominal distension and paralytic ileus may also occur in patients.[24]
Physical Examination
Common physical examination of patients with Neutropenic enterocolitis is usually remarkable for Abdominal discomfort which can be diffuse or localized, with the right lower quadrant being the most common location. A rigid abdomen could be an indication of bowel perforation.[25]
Laboratory Findings
Laboratory findings consistent with the diagnosis of typhlitis include neutropenia with absolute neutrophil count <500 cells/microL, thrombocytopenia ranged from 4000/pl to 120,000/pl.[26]
Ultrasound
- Ultrasound (US) may be helpful in the diagnosis of Neutropenic enterocolitis. Findings on an ultrasound suggestive of Neutropenic enterocolitis include circumferential wall thickening and prominent submucosa .[27]
X-ray
An x-ray may be helpful in the diagnosis of Typhlitis but nonspecific. Findings on an x-ray suggestive of Neutropenic enterocolitis include inflated cecum with dilated small bowel loops, can detect free air.[28]
CT Scan
- A computed tomography (CT) scan of the abdomen may be helpful in the diagnosis of Neutropenic enterocolitis. Findings on CT scan suggestive of Neutropenic enterocolitis include Intestinal wall thickening, mesenteric stranding, intestinal dilatation, pneumatosis, distention and circumferential thickening of the cecal wall.[29]
Treatment
Medical Therapy
- The mainstay of treatment for Neutropenic enterocolitis consists of both supportive therapy and antimicrobials[30]
- Supportive therapy for Neutropenic colitis include bowel rest with nasogastric suction, intravenous fluids, and, if necessary, parenteral nutrition.
- Empiric therapy for Neutropenic colitis depends on antimicrobial exposure, bacteremia and local resistance pattern.
- Pipericillin-tazobactum, carbapenam, or an antipseudomonal cephalosporin is recommended among patients who develop neutropenic colitis and vancomycin is considered in case of mucositis is suspected, which is against gram positive bacteria.[31][32]
Surgery
- All individuals with Neutropenic enterocolitis should seek surgical advice as soon as possible.[33]
- Surgery is usually reserved for patients with either bowel perforation, pneumoperitoneum, or persistent gastrointestinal bleeding.
Primary Prevention
Effective measures for the primary prevention of Neutropenic enterocolitis include early detection and treatment can help to avoid problems and improve outcomes in patients who have undergone intensive chemotherapy or a stem cell transplant.Treatment with G-CSF not only speeds recovery from neutropenia episodes that occur during chemotherapy, but it also reduces the risk of consequences including mucositis.[34]
Secondary Prevention
Effective measures for the secondary prevention include early surgical evaluation in the management of this condition, as it can be life-saving for some patients who present with a complicated Neutropenic enterocolitis[35]
References
- ↑ Katz, Julie A.; Mahoney, Donald H.; Fernbach, Donald J.; Wagner, Milton L.; Gresik, Mary V. (1990). "Typhlitis. An 18-year experience and postmortem review". Cancer. 65 (4): 1041–1047. doi:10.1002/1097-0142(19900215)65:4<1041::AID-CNCR2820650433>3.0.CO;2-A. ISSN 0008-543X.
- ↑ Urbach DR, Rotstein OD (1999). "Typhlitis". Can J Surg. 42 (6): 415–9. PMC 3795130. PMID 10593241.
- ↑ Cloutier RL (2009). "Neutropenic enterocolitis". Emerg Med Clin North Am. 27 (3): 415–22. doi:10.1016/j.emc.2009.04.002. PMID 19646645.
- ↑ Rodrigues FG, Dasilva G, Wexner SD (2017). "Neutropenic enterocolitis". World J Gastroenterol. 23 (1): 42–47. doi:10.3748/wjg.v23.i1.42. PMC 5221285. PMID 28104979.
- ↑ "StatPearls". ( ). 2021: . PMID 31869058.
- ↑ Rodrigues FG, Dasilva G, Wexner SD (2017). "Neutropenic enterocolitis". World J Gastroenterol. 23 (1): 42–47. doi:10.3748/wjg.v23.i1.42. PMC 5221285. PMID 28104979.
- ↑ Chakravarty, K.; Scott, D. G. I.; Mccann, B. G. (1992). "FATAL NEUTROPENIC ENTEROCOLITIS ASSOCIATED WITH SULPHASALAZINE THERAPY FOR RHEUMATOID ARTHRITIS". Rheumatology. 31 (5): 351–353. doi:10.1093/rheumatology/31.5.351. ISSN 1462-0324.
- ↑ Bibbo C, Barbieri RA, Deitch EA, Brolin RE (2000). "Neutropenic enterocolitis in a trauma patient during antibiotic therapy for osteomyelitis". J Trauma. 49 (4): 760–3. doi:10.1097/00005373-200010000-00029. PMID 11038099.
- ↑ Davila ML (2006). "Neutropenic enterocolitis". Curr Opin Gastroenterol. 22 (1): 44–7. doi:10.1097/01.mog.0000198073.14169.3b. PMID 16319675.
- ↑ Cardona Zorrilla AF, Reveiz Herault L, Casasbuenas A, Aponte DM, Ramos PL (2006). "Systematic review of case reports concerning adults suffering from neutropenic enterocolitis". Clin Transl Oncol. 8 (1): 31–8. doi:10.1007/s12094-006-0092-y. PMID 16632437.
- ↑ Czepiel J, Dróżdż M, Pituch H, Kuijper EJ, Perucki W, Mielimonka A; et al. (2019). "Clostridium difficile infection: review". Eur J Clin Microbiol Infect Dis. 38 (7): 1211–1221. doi:10.1007/s10096-019-03539-6. PMC 6570665 Check
|pmc=value (help). PMID 30945014. - ↑ Pillet S, Pozzetto B, Roblin X (2016). "Cytomegalovirus and ulcerative colitis: Place of antiviral therapy". World J Gastroenterol. 22 (6): 2030–45. doi:10.3748/wjg.v22.i6.2030. PMC 4726676. PMID 26877608.
- ↑ "StatPearls". 2021. PMID 31335045.
- ↑ Ramachandran V, Kolli SS, Strowd LC (2019). "Review of Graft-Versus-Host Disease". Dermatol Clin. 37 (4): 569–582. doi:10.1016/j.det.2019.05.014. PMID 31466596.
- ↑ Bhangu A, Søreide K, Di Saverio S, Assarsson JH, Drake FT (2015). "Acute appendicitis: modern understanding of pathogenesis, diagnosis, and management". Lancet. 386 (10000): 1278–1287. doi:10.1016/S0140-6736(15)00275-5. PMID 26460662.
- ↑ Theodoropoulou A, Koutroubakis IE (2008). "Ischemic colitis: clinical practice in diagnosis and treatment". World J Gastroenterol. 14 (48): 7302–8. doi:10.3748/wjg.14.7302. PMC 2778113. PMID 19109863.
- ↑ Gorschlüter M, Mey U, Strehl J, Ziske C, Schepke M, Schmidt-Wolf IG; et al. (2005). "Neutropenic enterocolitis in adults: systematic analysis of evidence quality". Eur J Haematol. 75 (1): 1–13. doi:10.1111/j.1600-0609.2005.00442.x. PMID 15946304.
- ↑ Aksoy DY, Tanriover MD, Uzun O, Zarakolu P, Ercis S, Ergüven S; et al. (2007). "Diarrhea in neutropenic patients: a prospective cohort study with emphasis on neutropenic enterocolitis". Ann Oncol. 18 (1): 183–189. doi:10.1093/annonc/mdl337. PMID 17023562.
- ↑ Nesher L, Rolston KV (2013). "Neutropenic enterocolitis, a growing concern in the era of widespread use of aggressive chemotherapy". Clin Infect Dis. 56 (5): 711–7. doi:10.1093/cid/cis998. PMID 23196957.
- ↑ Biasoli, I; Nucci, M; Spector, N; Portugal, R; Domingues, A; Pulcheri, W (1997). "Risk factors for typhlitis". Oncology Reports. doi:10.3892/or.4.5.1029. ISSN 1021-335X.
- ↑ Gorschlüter M, Mey U, Strehl J, Ziske C, Schepke M, Schmidt-Wolf IG; et al. (2005). "Neutropenic enterocolitis in adults: systematic analysis of evidence quality". Eur J Haematol. 75 (1): 1–13. doi:10.1111/j.1600-0609.2005.00442.x. PMID 15946304.
- Prognosis is generally poor, and the mortality rate of patients with Neutropenic enterocolitis is as high as 50% especially in patients with transmural inflammation or intestinal perforation
- ↑ Wade DS, Nava HR, Douglass HO (1992). "Neutropenic enterocolitis. Clinical diagnosis and treatment". Cancer. 69 (1): 17–23. doi:10.1002/1097-0142(19920101)69:1<17::aid-cncr2820690106>3.0.co;2-x. PMID 1727660.
- ↑ Sloas MM, Flynn PM, Kaste SC, Patrick CC (1993). "Typhlitis in children with cancer: a 30-year experience". Clin Infect Dis. 17 (3): 484–90. doi:10.1093/clinids/17.3.484. PMID 8218694.
- ↑ Nesher L, Rolston KV (2013). "Neutropenic enterocolitis, a growing concern in the era of widespread use of aggressive chemotherapy". Clin Infect Dis. 56 (5): 711–7. doi:10.1093/cid/cis998. PMID 23196957.
- ↑ Nesher, L.; Rolston, K. V. I. (2012). "Neutropenic Enterocolitis, a Growing Concern in the Era of Widespread Use of Aggressive Chemotherapy". Clinical Infectious Diseases. 56 (5): 711–717. doi:10.1093/cid/cis998. ISSN 1058-4838.
- ↑ Katz JA, Wagner ML, Gresik MV, Mahoney DH, Fernbach DJ (1990). "Typhlitis. An 18-year experience and postmortem review". Cancer. 65 (4): 1041–7. doi:10.1002/1097-0142(19900215)65:4<1041::aid-cncr2820650433>3.0.co;2-a. PMID 2404562.
- ↑ Tamburrini, Stefania; Setola, Francesca Rosa; Belfiore, Maria Paola; Saturnino, Pietro Paolo; Della Casa, Maria Gabriella; Sarti, Giuseppe; Abete, Roberta; Marano, Ines (2018). "Ultrasound diagnosis of typhlitis". Journal of Ultrasound. 22 (1): 103–106. doi:10.1007/s40477-018-0333-2. ISSN 1876-7931.
- ↑ Terzis JK, Daniel RK, Williams HB, Spencer PS (1978). "Benign fatty tumors of the peripheral nerves". Ann Plast Surg. 1 (2): 193–216. doi:10.1097/00000637-197803000-00011. PMID 727660.
- ↑ Kirkpatrick, Iain D. C.; Greenberg, Howard M. (2003). "Gastrointestinal Complications in the Neutropenic Patient: Characterization and Differentiation with Abdominal CT". Radiology. 226 (3): 668–674. doi:10.1148/radiol.2263011932. ISSN 0033-8419.
- ↑ Mullassery D, Bader A, Battersby AJ, Mohammad Z, Jones EL, Parmar C; et al. (2009). "Diagnosis, incidence, and outcomes of suspected typhlitis in oncology patients--experience in a tertiary pediatric surgical center in the United Kingdom". J Pediatr Surg. 44 (2): 381–5. doi:10.1016/j.jpedsurg.2008.10.094. PMID 19231539.
- ↑ Freifeld AG, Bow EJ, Sepkowitz KA, Boeckh MJ, Ito JI, Mullen CA; et al. (2011). "Clinical practice guideline for the use of antimicrobial agents in neutropenic patients with cancer: 2010 update by the infectious diseases society of america". Clin Infect Dis. 52 (4): e56–93. doi:10.1093/cid/cir073. PMID 21258094.
- ↑ Salazar R, Solá C, Maroto P, Tabernero JM, Brunet J, Verger G; et al. (1999). "Infectious complications in 126 patients treated with high-dose chemotherapy and autologous peripheral blood stem cell transplantation". Bone Marrow Transplant. 23 (1): 27–33. doi:10.1038/sj.bmt.1701520. PMID 10037047.
- ↑ Shamberger RC, Weinstein HJ, Delorey MJ, Levey RH (1986). "The medical and surgical management of typhlitis in children with acute nonlymphocytic (myelogenous) leukemia". Cancer. 57 (3): 603–9. doi:10.1002/1097-0142(19860201)57:3<603::aid-cncr2820570335>3.0.co;2-k. PMID 3484659.
- ↑ Steward WP (1993). "Granulocyte and granulocyte-macrophage colony-stimulating factors". Lancet. 342 (8864): 153–7. doi:10.1016/0140-6736(93)91350-u. PMID 7687319.
- ↑ Varki, Ajit P.; Armitage, James O.; Feagler, John R. (1979). "Typhlitis in acute leukemia.Successful treatment by early surgical intervention". Cancer. 43 (2): 695–697. doi:10.1002/1097-0142(197902)43:2<695::AID-CNCR2820430242>3.0.CO;2-9. ISSN 0008-543X.