Thrombus: Difference between revisions
| Line 31: | Line 31: | ||
==Venous thrombus== | ==Venous thrombus== | ||
The pathophysiology of venous thrombosis or thromboembolism is associated to plasma [[hypercoagulability]] triggered by the expression of procoagulant activity, in intact [[endothelium]], from inflammation or reduced/static [[blood flow]] resulting in prolonged immobility. Therefore the venous clots are slowly formed involving the formation of fibrin-rich “red clots” which have regions or layers showing substantial [[erythrocyte]] incorporation. Finally, the venous thrombi may initiate behind valve pockets, in which the reduced or static flow decreases wall shear stress that normally regulates endothelial cell phenotype. Thus, this phenomenum makes the venous endotelium more likely to develop inappropriate expression of intravascular procoagulant activity and so to trigger [[venous thromboembolism]].<ref name="Machlus-2011">{{Cite journal | last1 = Machlus | first1 = KR. | last2 = Cardenas | first2 = JC. | last3 = Church | first3 = FC. | last4 = Wolberg | first4 = AS. | title = Causal relationship between hyperfibrinogenemia, thrombosis, and resistance to thrombolysis in mice. | journal = Blood | volume = 117 | issue = 18 | pages = 4953-63 | month = May | year = 2011 | doi = 10.1182/blood-2010-11-316885 | PMID = 21355090 }}</ref><ref name="Campbell-2009">{{Cite journal | last1 = Campbell | first1 = RA. | last2 = Overmyer | first2 = KA. | last3 = Selzman | first3 = CH. | last4 = Sheridan | first4 = BC. | last5 = Wolberg | first5 = AS. | title = Contributions of extravascular and intravascular cells to fibrin network formation, structure, and stability. | journal = Blood | volume = 114 | issue = 23 | pages = 4886-96 | month = Nov | year = 2009 | doi = 10.1182/blood-2009-06-228940 | PMID = 19797520 }}</ref> | The pathophysiology of venous thrombosis or thromboembolism is associated to plasma [[hypercoagulability]] triggered by the expression of procoagulant activity, in intact [[endothelium]], from inflammation or reduced/static [[blood flow]] resulting in prolonged immobility. Therefore the venous clots are slowly formed involving the formation of fibrin-rich “red clots” which have regions or layers showing substantial [[erythrocyte]] incorporation. Finally, the venous thrombi may initiate behind valve pockets, in which the reduced or static flow decreases wall shear stress that normally regulates endothelial cell phenotype.<ref name="Wolberg-2012">{{Cite journal | last1 = Wolberg | first1 = AS. | last2 = Aleman | first2 = MM. | last3 = Leiderman | first3 = K. | last4 = Machlus | first4 = KR. | title = Procoagulant activity in hemostasis and thrombosis: Virchow's triad revisited. | journal = Anesth Analg | volume = 114 | issue = 2 | pages = 275-85 | month = Feb | year = 2012 | doi = 10.1213/ANE.0b013e31823a088c | PMID = 22104070 }}</ref> Thus, this phenomenum makes the venous endotelium more likely to develop inappropriate expression of intravascular procoagulant activity and so to trigger [[venous thromboembolism]].<ref name="Machlus-2011">{{Cite journal | last1 = Machlus | first1 = KR. | last2 = Cardenas | first2 = JC. | last3 = Church | first3 = FC. | last4 = Wolberg | first4 = AS. | title = Causal relationship between hyperfibrinogenemia, thrombosis, and resistance to thrombolysis in mice. | journal = Blood | volume = 117 | issue = 18 | pages = 4953-63 | month = May | year = 2011 | doi = 10.1182/blood-2010-11-316885 | PMID = 21355090 }}</ref><ref name="Campbell-2009">{{Cite journal | last1 = Campbell | first1 = RA. | last2 = Overmyer | first2 = KA. | last3 = Selzman | first3 = CH. | last4 = Sheridan | first4 = BC. | last5 = Wolberg | first5 = AS. | title = Contributions of extravascular and intravascular cells to fibrin network formation, structure, and stability. | journal = Blood | volume = 114 | issue = 23 | pages = 4886-96 | month = Nov | year = 2009 | doi = 10.1182/blood-2009-06-228940 | PMID = 19797520 }}</ref> | ||
==See also== | ==See also== | ||
Revision as of 16:55, 15 November 2013
|
WikiDoc Resources for Thrombus |
|
Articles |
|---|
|
Most recent articles on Thrombus |
|
Media |
|
Evidence Based Medicine |
|
Clinical Trials |
|
Ongoing Trials on Thrombus at Clinical Trials.gov Clinical Trials on Thrombus at Google
|
|
Guidelines / Policies / Govt |
|
US National Guidelines Clearinghouse on Thrombus
|
|
Books |
|
News |
|
Commentary |
|
Definitions |
|
Patient Resources / Community |
|
Directions to Hospitals Treating Thrombus Risk calculators and risk factors for Thrombus
|
|
Healthcare Provider Resources |
|
Causes & Risk Factors for Thrombus |
|
Continuing Medical Education (CME) |
|
International |
|
|
|
Business |
|
Experimental / Informatics |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]
Overview
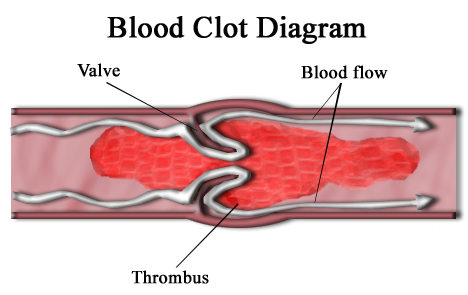
A thrombus, or blood clot, is the final product of the blood coagulation step in hemostasis. It is achieved via the aggregation of platelets that form a platelet plug, and the activation of the humoral coagulation system (i.e. clotting factors). A thrombus is physiologic in cases of injury, but pathologic in case of thrombosis.
Pathophysiology
Specifically, a thrombus is a blood clot in an intact blood vessel. A thrombus in a large blood vessel will decrease blood flow through that vessel. In a small blood vessel, blood flow may be completely cut-off resulting in death of tissue supplied by that vessel. If a thrombus dislodges and becomes free-floating, it is an embolus.
Some of the conditions which elevate risk of blood clots developing include atrial fibrillation (a form of cardiac arrhythmia), heart valve replacement, a recent heart attack, extended periods of inactivity (see deep venous thrombosis), and genetic or disease-related deficiencies in the blood's clotting abilities.
Preventing blood clots reduces the risk of stroke, heart attack and pulmonary embolism. Heparin and warfarin are often used to inhibit the formation and growth of existing blood clots, thereby allowing the body to shrink and dissolve the blood clots through normal methods (see anticoagulant).
A thrombus differs from a hematoma by:
- Having high hematocrit
- Being non-laminar
- Being soft and friable
- Having an absence of circulation
Virchow's Triad describes the conditions necessary for thrombus formation:
- Changes in vessel wall morphology (e.g. trauma, atheroma)
- Changes in blood flow through the vessel (e.g. valvulitis, aneurysm)
- Changes in blood composition (e.g. leukaemia, hypercoagulability disorders)
Disseminated intravascular coagulation (DIC) involves widespread microthrombi formation throughout the majority of the blood vessels. This is due to excessive consumption of coagulation factors and fibrinolysis using all of the body's available platelets and clotting factors. The end result is ischaemic necrosis of the affected tissue/organs and spontaneous bleeding due to the lack of clotting factors. Causes are septicaemia, acute leukaemia, shock, snake bites or severe trauma. Treatment involves the use of fresh, frozen plasma to restore the level of clotting factors in the blood.
Arterial thrombus
The pathogenic process of arterial thrombosis involves the formation of platelet-rich “white clots” after the rupture of atherosclerotic plaques and exposure of procoagulant material such as lipid-rich macrophages (foam cells), collagen, tissue factor, and/or endothelial breach, in a high shear environment. The exposed material come from within the plaque and also from the activation and aggregation of platelets. Platelet accumulation and fibrin deposition cause an occlusive platelet-rich intravascular thrombus. The growing thrombus increases the degree of narrowing, which may result in extremely high shear rates within the stenotic region. This phenomenum is responsible for a turbulent flow which is developed downstream of the stenosis depending on stenosis geometry and location in the vasculature.[1][2]
Venous thrombus
The pathophysiology of venous thrombosis or thromboembolism is associated to plasma hypercoagulability triggered by the expression of procoagulant activity, in intact endothelium, from inflammation or reduced/static blood flow resulting in prolonged immobility. Therefore the venous clots are slowly formed involving the formation of fibrin-rich “red clots” which have regions or layers showing substantial erythrocyte incorporation. Finally, the venous thrombi may initiate behind valve pockets, in which the reduced or static flow decreases wall shear stress that normally regulates endothelial cell phenotype.[2] Thus, this phenomenum makes the venous endotelium more likely to develop inappropriate expression of intravascular procoagulant activity and so to trigger venous thromboembolism.[3][4]
See also
- Air pollution
- Embolism
- Thrombolysis ("Destruction of clot")
- Thrombogenicity (the tendency to clot)
References
- ↑ Bark, DL.; Ku, DN. (2010). "Wall shear over high degree stenoses pertinent to atherothrombosis". J Biomech. 43 (15): 2970–7. doi:10.1016/j.jbiomech.2010.07.011. PMID 20728892. Unknown parameter
|month=ignored (help) - ↑ 2.0 2.1 Wolberg, AS.; Aleman, MM.; Leiderman, K.; Machlus, KR. (2012). "Procoagulant activity in hemostasis and thrombosis: Virchow's triad revisited". Anesth Analg. 114 (2): 275–85. doi:10.1213/ANE.0b013e31823a088c. PMID 22104070. Unknown parameter
|month=ignored (help) - ↑ Machlus, KR.; Cardenas, JC.; Church, FC.; Wolberg, AS. (2011). "Causal relationship between hyperfibrinogenemia, thrombosis, and resistance to thrombolysis in mice". Blood. 117 (18): 4953–63. doi:10.1182/blood-2010-11-316885. PMID 21355090. Unknown parameter
|month=ignored (help) - ↑ Campbell, RA.; Overmyer, KA.; Selzman, CH.; Sheridan, BC.; Wolberg, AS. (2009). "Contributions of extravascular and intravascular cells to fibrin network formation, structure, and stability". Blood. 114 (23): 4886–96. doi:10.1182/blood-2009-06-228940. PMID 19797520. Unknown parameter
|month=ignored (help)
External links
- Treatment and Symptoms of Blood Clots -- Med-Help.net, Medical Information Resource, 1999
- North American Thrombosis Forum - NATF is a nonprofit organization that aims to promote public education, policy and advocacy for clotting diseases of the cardiovascular system.
- Air Pollution Triggers Blood Clots - US Study
- Cleveland Clinic Web Chat - Blood Clots, Hypercoagulable States, DVT with Dr. John Bartholomew
ar:جلطة de:Thrombus eo:Trombo it:Trombo lt:Trombas hu:Thrombus qu:Sirk'a unquy sv:Blodpropp