Terazosin
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: João André Alves Silva, M.D. [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Terazosin is a alpha-adrenergic blocker that is FDA approved for the {{{indicationType}}} of symptomatic benign prostatic hyperplasia (BPH) and hypertension. Common adverse reactions include orthostatic hypotension, palpitations, peripheral edema, nausea, asthenia, dizziness headache, somnolence and nasal congestion.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Benign Prostatic Hyperplasia
- Initial dose :
- 1 mg at bedtime is the starting dose for all patients (should not be exceeded as an initial dose).
- Patients should be closely followed during initial administration in order to minimize the risk of severe hypotensive response.
- Subsequent doses :
- The initial dose should be increased in a stepwise fashion to 2 mg, 5 mg, or 10 mg once daily to achieve the desired improvement of symptoms and/or flow rates.
- Doses of 10 mg once daily are generally required for the clinical response.
- Treatment with 10 mg for a minimum of 4 to 6 weeks may be required to assess whether a beneficial response has been achieved. Some patients may not achieve a clinical response despite appropriate titration. :* :* Some patients responded at a 20 mg daily dose, however, there was an insufficient number of patients studied to draw definitive conclusions about this dose.
- If terazosin administration is discontinued for several days or longer, therapy should be reinstituted using the initial dosing regimen.
Hypertension
- Initial dose:
- 1 mg at bedtime is the starting dose for all patients (this dose should not be exceeded). This initial dosing regimen should be strictly observed to minimize the potential for severe hypotensive effects.
- Subsequent doses:
- The dose may be slowly increased to achieve the desired blood pressure response.
- The usual recommended dose range is 1 mg to 5 mg administered once a day
- Some patients may benefit from doses as high as 20 mg per day.
- Doses over 20 mg do not appear to provide further blood pressure effect and doses over 40 mg have not been studied.
- Blood pressure should be monitored at the end of the dosing interval to be sure control is maintained throughout the interval.
- If response is substantially diminished at 24 hours an increased dose or use of a twice daily regimen can be considered.
- If terazosin administration is discontinued for several days or longer, therapy should be reinstituted using the initial dosing regimen.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of terazosin in adult patients.
Non–Guideline-Supported Use
Chronic prostatitis - chronic pelvic pain syndrome
- Dosing Information
- (Dosage)
Hyperlipidemia
- Dosing Information
- (Dosage)
Nocturia
- Dosing Information
- (Dosage)
Oligozoospermia
- Dosing Information
- (Dosage)
Radiation-induced disorder - Urethritis
- Dosing Information
- (Dosage)
Spinal cord injury
- Dosing Information
- (Dosage)
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
There is limited information regarding FDA-label Guideline-Supported Use of terazosin in pediatric patients.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of terazosin in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non-Guideline-Supported Use of terazosin in pediatric patients.
Contraindications
- Terazosin capsules are contraindicated in patients known to be hypersensitive to terazosin hydrochloride.
Warnings
Syncope and “first-dose” effect
- Terazosin capsules, like other alpha-adrenergic blocking agents, can cause marked lowering of blood pressure, especially postural hypotension, and syncope in association with the first dose or first few days of therapy. A similar effect can be anticipated if therapy is interrupted for several days and then restarted.
- Syncope has also been reported with other alpha-adrenergic blocking agents in association with rapid dosage increases or the introduction of another antihypertensive drug.
- Syncope is believed to be due to an excessive postural hypotensive effect, although occasionally the syncopal episode has been preceded by a bout of severe supraventricular tachycardia with heart rates of 120 to 160 beats per minute. Additionally, the possibility of the contribution of hemodilution to the symptoms of postural hypotension should be considered.
- To decrease the likelihood of syncope or excessive hypotension, treatment should:
- Always be initiated with a 1 mg dose of terazosin capsules, given at bedtime.
- The 2 mg, 5 mg and 10 mg capsules are not indicated as initial therapy.
- Dosage should then be increased slowly, according to recommendations in the Dosage and Administration section and additional antihypertensive agents should be added with caution.
- The patient should be cautioned to avoid situations, such as driving or hazardous tasks, where injury could result should syncope occur during initiation of therapy.
In early investigational studies, where increasing single doses up to 7.5 mg were given at 3 day intervals, tolerance to the first dose phenomenon did not necessarily develop and the “first-dose” effect could be observed at all doses. Syncopal episodes occurred in 3 of the 14 subjects given terazosin at doses of 2.5, 5 and 7.5 mg, which are higher than the recommended initial dose; in addition, severe orthostatic hypotension (blood pressure falling to 50/0 mmHg) was seen in two others and dizziness, tachycardia, and lightheadedness occurred in most subjects. These adverse effects all occurred within 90 minutes of dosing. In three placebo-controlled BPH studies 1, 2, and 3, the incidence of postural hypotension in the terazosin treated patients was 5.1%, 5.2%, and 3.7% respectively. In multiple dose clinical trials involving nearly 2000 hypertensive patients treated with terazosin capsules, syncope was reported in about 1% of patients. Syncope was not necessarily associated only with the first dose. If syncope occurs, the patient should be placed in a recumbent position and treated supportively as necessary. There is evidence that the orthostatic effect of terazosin is greater, even in chronic use, shortly after dosing. The risk of the events is greatest during the initial seven days of treatment, but continues at all time intervals.
Priapism
- Rarely, terazosin and other α1-antagonists have been associated with priapism (painful penile erection, sustained for hours and unrelieved by sexual intercourse or masturbation). Two or three dozen cases have been reported.
- Since this condition can lead to permanent impotence if not promptly treated, patients must be advised about the seriousness of the condition.
Adverse Reactions
Clinical Trials Experience
Benign Prostatic Hyperplasia
- The incidence of treatment-emergent adverse events has been ascertained clinical trials conducted worldwide.
- All adverse events reported during these trials were recorded as adverse reactions.
- The incidence rates presented below are based on combined data from six placebo-controlled trials involving once-a-day administration of terazosin at doses ranging from 1 to 20 mg. Table 1 summarizes those adverse events reported for patients in these trials when the incidence rate in the terazosin group was at least 1%, and was greater than that for the placebo group, or where the reaction is of clinical interest. Asthenia, postural hypotension, dizziness, somnolence, nasal congestion/rhinitis, and impotence were the only events that were significantly (p ≤ 0.05) more common in patients receiving terazosin than in patients receiving placebo.
- The incidence of urinary tract infection was significantly lower in the patients receiving terazosin than in patients receiving placebo. An analysis of the incidence rate of hypotensive adverse events adjusted for the length of drug treatment has shown that the risk of the events is greatest during the initial seven days of treatment, but continues at all time intervals.

- Additional adverse events have been reported, but these are, in general, not distinguishable from symptoms that might have occurred in the absence of exposure to terazosin. The safety profile of patients treated in the long-term open-label study was similar to that observed in the controlled studies.
- The adverse events were usually transient and mild or moderate in intensity, but sometimes were serious enough to interrupt treatment. In the placebo-controlled clinical trials, the rates of premature termination due to adverse events were not statistically different between the placebo and terazosin groups. The adverse events that were bothersome, as judged by their being reported as reasons for discontinuation of therapy by at least 0.5% of the terazosin group and being reported more often than in the placebo group, are shown in Table 2.
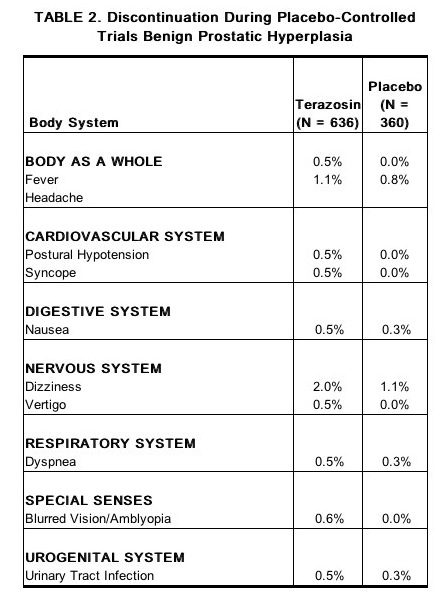
Hypertension
- The prevalence of adverse reactions has been ascertained from clinical trials conducted primarily in the United States. All adverse experiences (events) reported during these trials were recorded as adverse reactions. The prevalence rates presented below are based on combined data from fourteen placebo-controlled trials involving once-a-day administration of terazosin, as monotherapy or in combination with other antihypertensive agents, at doses ranging from 1 to 40 mg. Table 3 summarizes those adverse experiences reported for patients in these trials where the prevalence rate in the terazosin group was at least 5%, where the prevalence rate for the terazosin group was at least 2% and was greater than the prevalence rate for the placebo group, or where the reaction is of particular interest.
- Asthenia, blurred vision, dizziness, nasal congestion, nausea, peripheral edema, palpitations and somnolence were the only symptoms that were significantly (p < 0.05) more common in patients receiving terazosin than in patients receiving placebo.
- Similar adverse reaction rates were observed in placebo-controlled monotherapy trials.

- Additional adverse reactions have been reported, but these are, in general, not distinguishable from symptoms that might have occurred in the absence of exposure to terazosin. The following additional adverse reactions were reported by at least 1% of 1987 patients who received terazosin in controlled or open, short- or long-term clinical trials or have been reported during marketing experience:
Central Nervous System
Cardiovascular
Respiratory
- Bronchitis
- Cold symptoms
- Epistaxis
- Flu symptoms
- Increased cough
- Pharyngitis
- Rhinitis
Gastrointestinal
Miscellaneous
- The adverse reactions were usually mild or moderate in intensity but sometimes were serious enough to interrupt treatment.
- The adverse reactions that were most bothersome, as judged by their being reported as reasons for discontinuation of therapy by at least 0.5% of the terazosin group and being reported more often than in the placebo group, are shown in Table 4.
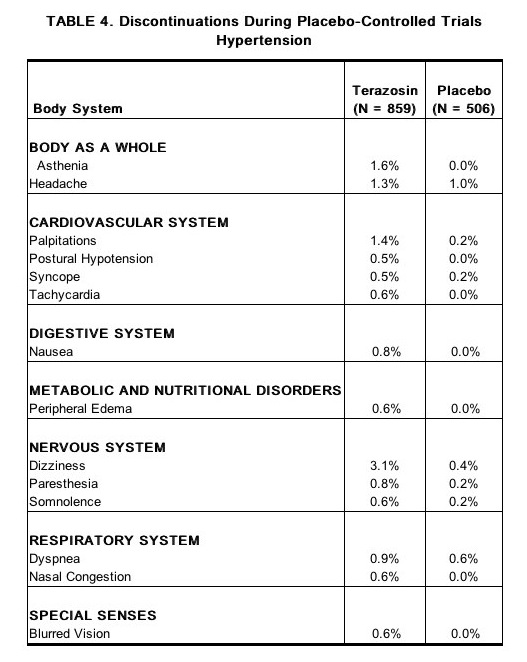
Postmarketing Experience
- Post-marketing experience indicates that in rare instances patients may develop allergic reactions, including anaphylaxis, following administration of terazosin hydrochloride.
- There have been reports of priapism and thrombocytopenia during post-marketing surveillance.
- Atrial fibrillation has also been reported.
- During cataract surgery, a variant of small pupil syndrome known as Intraoperative floppy iris syndrome (IFIS) has been reported in association with alpha-1 blocker therapy.
Drug Interactions
- In controlled trials, terazosin have been added to diuretics, and several beta-adrenergic blockers; no unexpected interactions were observed.
- Terazosin has also been used in patients on a variety of concomitant therapies; while these were not formal interaction studies, no interactions were observed. Terazosin has been used concomitantly in at least 50 patients on the following drugs or drug classes:
- Cardiovascular agents:
- Corticosteroids
- Gastrointestinal agents
- In a study (n=24) where terazosin and verapamil were administered concomitantly, terazosin’s mean AUC0-24 increased 11% after the first verapamil dose and after 3 weeks of verapamil treatment it increased by 24% with associated increases in Cmax (25%) and Cmin (32%) means. Terazosin mean Tmax decreased from 1.3 hours to 0.8 hours after 3 weeks of verapamil treatment. Statistically significant differences were not found in the verapamil level with and without terazosin.
- In a study (n=6) where terazosin and captopril were administered concomitantly, plasma disposition of captopril was not influenced by concomitant administration of terazosin and terazosin maximum plasma concentrations increased linearly with dose at steady-state after administration of terazosin plus captopril.
Use in Specific Populations
Pregnancy
- Teratogenic Effects :
- Terazosin capsules were not teratogenic in either rats or rabbits when administered at oral doses up to 280 and 60 times, respectively, the maximum recommended human dose.
- Fetal resorptions occurred in rats dosed with 480 mg/kg/day, approximately 280 times the maximum recommended human dose.
- Increased fetal resorptions, decreased fetal weight and an increased number of supernumerary ribs were observed in offspring of rabbits dosed with 60 times the maximum recommended human dose.
- These findings (in both species) were most likely secondary to maternal toxicity.
There are no adequate and well-controlled studies in pregnant women and the safety of terazosin in pregnancy has not been established. Terazosin capsules are not recommended during pregnancy unless the potential benefit justifies the potential risk to the mother and fetus.
- Nonteratogenic Effects :
- In a peri- and post-natal development study in rats, significantly more pups died in the group dosed with 120 mg/kg/day (> 75 times the maximum recommended human dose) than in the control group during the three-week postpartum period.
- There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of terazosin in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Terazosin during labor and delivery.
Nursing Mothers
- It is not known whether terazosin is excreted in breast milk.
- Because many drugs are excreted in breast milk, caution should be exercised when terazosin capsules are administered to a nursing woman.
Pediatric Use
- Safety and effectiveness in pediatric patients have not been determined.
Geriatic Use
There is no FDA guidance on the use of Terazosin in geriatric settings.
Gender
There is no FDA guidance on the use of Terazosin with respect to specific gender populations.
Race
There is no FDA guidance on the use of Terazosin with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Terazosin in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Terazosin in patients with hepatic impairment.
Females of Reproductive Potential and Males
- Terazosin was devoid of mutagenic potential when evaluated in vivo and in vitro (the Ames test, in vivo cytogenetics, the dominant lethal test in mice, in vivo Chinese hamster chromosome aberration test and V79 forward mutation assay).
- Terazosin administered in the feed to rats at doses of 8, 40, and 250 mg/kg/day (70, 350, and 2100 mg/M2/day), for two years, was associated with a statistically significant increase in benign adrenal medullary tumors of male rats exposed to the 250 mg/kg dose. This dose is 175 times the maximum recommended human dose of 20 mg (12 mg/M2). Female rats were unaffected.
- Terazosin was not oncogenic in mice when administered in feed for 2 years at a maximum tolerated dose of 32 mg/kg/day (110 mg/M2; 9 times the maximum recommended human dose).
- The absence of mutagenicity in a battery of tests, of tumorigenicity of any cell type in the mouse carcinogenicity assay, of increased total tumor incidence in either species, and of proliferative adrenal lesions in female rats, suggests a male rat species-specific event. Numerous other diverse pharmaceutical and chemical compounds have also been associated with benign adrenal medullary tumors in male rats without supporting evidence for carcinogenicity in man.
- The effect of terazosin on fertility was assessed in a standard fertility/reproductive performance study in which male and female rats were administered oral doses of 8, 30 and 120 mg/kg/day. Four of 20 male rats given 30 mg/kg (240 mg/M2; 20 times the maximum recommended human dose) and five of 19 male rats given 120 mg/kg (960 mg/M2; 80 times the maximum recommended human dose) failed to sire a litter. Testicular weights and morphology were unaffected by treatment. Vaginal smears at 30 and 120 mg/kg/day, however, appeared to contain less sperm than smears from control matings and good correlation was reported between sperm count and subsequent pregnancy.
- Oral administration of terazosin for one or two years elicited a statistically significant increase in the incidence of testicular atrophy in rats exposed to 40 and 250 mg/kg/day (29 and 175 times the maximum recommended human dose), but not in rats exposed to 8 mg/kg/day (> 6 times the maximum recommended human dose). Testicular atrophy was also observed in dogs dosed with 300 mg/kg/day (> 500 times the maximum recommended human dose) for three months but not after one year when dosed with 20 mg/kg/day (38 times the maximum recommended human dose). This lesion has also been seen with Minipress®, another (marketed) selective-alpha-1 blocking agent.
Immunocompromised Patients
There is no FDA guidance one the use of Terazosin in patients who are immunocompromised.
Administration and Monitoring
Administration
Oral
Monitoring
Syncope and “First-dose” Effect
- Terazosin capsules, like other alpha-adrenergic blocking agents, can cause marked lowering of blood pressure, especially postural hypotension, and syncope in association with the first dose or first few days of therapy. Blood pressure should be monitored, particularly when increasing the dosage of terazosin, in order to avoid syncope.
Benign prostatic hyperplasia
- A patient taking terazosin should see an effect on his symptoms in 2 to 4 weeks. Therefore, continuous check-ups with the physician, to evaluate the progress regarding the BPH and monitor the blood pressure should be done, in addition to other regular check-ups.
IV Compatibility
There is limited information regarding the compatibility of Terazosin and IV administrations.
Overdosage
Acute Overdose
- In case of overdosage of terazosin capsules leading to:
Management
- Support of the cardiovascular system is of first importance.
- Restoration of blood pressure and normalization of heart rate may be accomplished by keeping the patient in the supine position.
- If this measure is inadequate, shock should first be treated with volume expanders.
- If necessary, vasopressors should then be used and renal function should be monitored and supported as needed.
- Laboratory data indicate that terazosin is 90-94% protein bound; therefore, dialysis may not be of benefit.
Pharmacology
| Template:Px | |
Terazosin
| |
| Systematic (IUPAC) name | |
| (RS)-6,7-dimethoxy-2-[4-(tetrahydrofuran-2-ylcarbonyl)piperazin-1-yl]quinazolin-4-amine | |
| Identifiers | |
| CAS number | |
| ATC code | G04 |
| PubChem | |
| DrugBank | |
| Chemical data | |
| Formula | Template:OrganicBox atomTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox |
| Mol. mass | 387.433 g/mol |
| SMILES | & |
| Synonyms | [4-(4-amino-6,7-dimethoxy-quinazolin-2-yl)piperazin-1-yl]-tetrahydrofuran-2-yl-methanone |
| Pharmacokinetic data | |
| Bioavailability | ? |
| Protein binding | 90-94% |
| Metabolism | ? |
| Half life | 12 hours |
| Excretion | ? |
| Therapeutic considerations | |
| Pregnancy cat. |
? |
| Legal status |
?(US) |
| Routes | ? |
Mechanism of Action
(Description)
Structure
(Description with picture)
Pharmacodynamics
(Description)
Pharmacokinetics
(Description)
Nonclinical Toxicology
(Description)
Clinical Studies
There is limited information regarding Clinical Studies of terazosin in the drug label.
How Supplied
(Description)
Storage
There is limited information regarding Terazosin Storage in the drug label.
Images
Drug Images
{{#ask: Page Name::Terazosin |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
{{#ask: Label Page::Terazosin |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
(Patient Counseling Information)
Precautions with Alcohol
Alcohol-Terazosin interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
- Hytrin
Look-Alike Drug Names
- (Paired Confused Name 1a) — (Paired Confused Name 1b)
- (Paired Confused Name 2a) — (Paired Confused Name 2b)
- (Paired Confused Name 3a) — (Paired Confused Name 3b)
Drug Shortage Status
Drug Shortage
Price
References
The contents of this FDA label are provided by the National Library of Medicine.