Rivaroxaban: Difference between revisions
No edit summary |
No edit summary |
||
| Line 336: | Line 336: | ||
*Switching from rivaroxaban to [[warfarin ]]- No clinical trial data are available to guide converting patients from rivaroxaban to [[warfarin]]. rivaroxaban affects [[INR]], so [[INR]] measurements made during coadministration with [[warfarin]] may not be useful for determining the appropriate dose of warfarin. One approach is to discontinue rivaroxaban and begin both a parenteral [[anticoagulant]] and [[warfarin ]]at the time the next dose of rivaroxaban would have been taken. | *Switching from rivaroxaban to [[warfarin ]]- No clinical trial data are available to guide converting patients from rivaroxaban to [[warfarin]]. rivaroxaban affects [[INR]], so [[INR]] measurements made during coadministration with [[warfarin]] may not be useful for determining the appropriate dose of warfarin. One approach is to discontinue rivaroxaban and begin both a parenteral [[anticoagulant]] and [[warfarin ]]at the time the next dose of rivaroxaban would have been taken. | ||
*Switching from rivaroxaban to [[anticoagulants]] other than | *Switching from rivaroxaban to [[anticoagulants]] other than [[warfarin]] - For patients currently taking rivaroxaban and transitioning to an anticoagulant with rapid onset, discontinue rivaroxaban and give the first dose of the other anticoagulant (oral or parenteral) at the time that the next rivaroxaban dose would have been taken. | ||
* Switching from anticoagulants other than warfarin to rivaroxaban - For patients currently receiving an anticoagulant other than [[warfarin]], start rivaroxaban 0 to 2 hours prior to the next scheduled evening administration of the drug (e.g., [[low molecular weight heparin]] or non-warfarin oral anticoagulant) and omit administration of the other anticoagulant. For unfractionated heparin being administered by continuous infusion, stop the infusion and start rivaroxaban at the same time. | * Switching from anticoagulants other than warfarin to rivaroxaban - For patients currently receiving an anticoagulant other than [[warfarin]], start rivaroxaban 0 to 2 hours prior to the next scheduled evening administration of the drug (e.g., [[low molecular weight heparin]] or non-warfarin oral anticoagulant) and omit administration of the other anticoagulant. For unfractionated heparin being administered by continuous infusion, stop the infusion and start rivaroxaban at the same time. | ||
Revision as of 19:25, 2 July 2014
{{DrugProjectFormSinglePage
|authorTag=Alejandro Lemor, M.D. [1]
|genericName=Rivaroxaban
|aOrAn=a
|drugClass=Factor Xa inhibitor
|indication=reduction in the risk of stroke and systemic embolism in patients with nonvalvular atrial fibrillation, treatment of deep vein thrombosis (DVT), pulmonary embolism (PE), reduction in the risk of recurrence of DVT and of PE, and for the prophylaxis of DVT, which may lead to PE in patients undergoing knee or hip replacement surgery.
|hasBlackBoxWarning=Yes
|adverseReactions=bleeding
|blackBoxWarningTitle=PREMATURE DISCONTINUATION OF RIVAROXABAN INCREASES THE RISK OF THROMBOTIC EVENTS,
SPINAL/EPIDURAL HEMATOMA
|blackBoxWarningBody=PREMATURE DISCONTINUATION OF RIVAROXABAN INCREASES THE RISK OF THROMBOTIC EVENTS: Premature discontinuation of any oral anticoagulant, including rivaroxaban, increases the risk of thrombotic events. If anticoagulation with rivaroxaban is discontinued for a reason other than pathological bleeding or completion of a course of therapy, consider coverage with another anticoagulant.
SPINAL/EPIDURAL HEMATOMA: Epidural or spinal hematomas have occurred in patients treated with rivaroxaban who are receiving neuraxial anesthesia or undergoing spinal puncture. These hematomas may result in long-term or permanent paralysis. Consider these risks when scheduling patients for spinal procedures. Factors that can increase the risk of developing epidural or spinal hematomas in these patients include:
- Use of indwelling epidural catheters
- Concomitant use of other drugs that affect hemostasis, such as non-steroidal anti-inflammatory drugs (NSAIDs), platelet inhibitors, other anticoagulants
- A history of traumatic or repeated epidural or spinal punctures
- A history of spinal deformity or spinal surgery.
- Optimal timing between the administration of rivaroxaban and neuraxial procedures is not known
Monitor patients frequently for signs and symptoms of neurological impairment. If neurological compromise is noted, urgent treatment is necessary.
Consider the benefits and risks before neuraxial intervention in patients anticoagulated or to be anticoagulated for thromboprophylaxis |fdaLIADAdult=====Nonvalvular Atrial Fibrillation====
- For patients with creatinine clearance (CrCl) >50 mL/min, the recommended dose of rivaroxaban is 20 mg taken orally once daily with the evening meal. For patients with CrCl 15 to 50 mL/min, the recommended dose is 15 mg once daily with the evening meal.
Treatment of Deep Vein Thrombosis (DVT), Pulmonary Embolism (PE), and Reduction in the Risk of Recurrence of DVT and of PE
- The recommended dose of rivaroxaban for the initial treatment of acute DVT and/or PE is 15 mg taken orally twice daily with food for the first 21 days.
- After this initial treatment period, the recommended dose of rivaroxaban is 20 mg taken orally once daily with food, at approximately the same time each day.
- The recommended dose of rivaroxaban for reduction in the risk of recurrence of DVT or PE is 20 mg taken orally once daily with food at approximately the same time each day.
Prophylaxis of Deep Vein Thrombosis Following Hip or Knee Replacement Surgery
- The recommended dose of rivaroxaban is 10 mg taken orally once daily with or without food.
- The initial dose should be taken 6 to 10 hours after surgery provided that hemostasis has been established.
- For patients undergoing hip replacement surgery, treatment duration of 35 days is recommended.
- For patients undergoing knee replacement surgery, treatment duration of 12 days is recommended.
|offLabelAdultGuideSupport======Condition 1=====
- Developed by: (Organisation)
- Class of Recommendation: (Class) (Link)
- Strength of Evidence: (Category A/B/C) (Link)
- Dosing Information/Recommendation
- (Dosage)
Condition 2
- Developed by: (Organisation)
- Class of Recommendation: (Class) (Link)
- Strength of Evidence: (Category A/B/C) (Link)
- Dosing Information/Recommendation
- (Dosage)
|offLabelAdultNoGuideSupport======Condition 1=====
- Dosing Information
- (Dosage)
Condition 2
- Dosing Information
- (Dosage)
Condition 3
- Dosing Information
- (Dosage)
|fdaLIADPed======Condition 1=====
- Dosing Information
- (Dosage)
Condition 2
- Dosing Information
- (Dosage)
|offLabelPedGuideSupport======Condition 1=====
- Developed by: (Organisation)
- Class of Recommendation: (Class) (Link)
- Strength of Evidence: (Category A/B/C) (Link)
- Dosing Information/Recommendation
- (Dosage)
Condition 2
- Developed by: (Organisation)
- Class of Recommendation: (Class) (Link)
- Strength of Evidence: (Category A/B/C) (Link)
- Dosing Information/Recommendation
- (Dosage)
|offLabelPedNoGuideSupport======Condition 1=====
- Dosing Information
- (Dosage)
Condition 2
- Dosing Information
- (Dosage)
Condition 3
- Dosing Information
- (Dosage)
|contraindications=*Active pathological bleeding
- Severe hypersensitivity reaction to rivaroxaban
|warnings=====Increased Risk of Thrombotic Events after Premature Discontinuation====
- Premature discontinuation of any oral anticoagulant, including rivaroxaban, in the absence of adequate alternative anticoagulation increases the risk of thrombotic events.
- An increased rate of stroke was observed during the transition from rivaroxaban to warfarin in clinical trials in atrial fibrillation patients.
- If rivaroxaban is discontinued for a reason other than pathological bleeding or completion of a course of therapy, consider coverage with another anticoagulant.
Risk of Bleeding
- Rivaroxaban increases the risk of bleeding and can cause serious or fatal bleeding. In deciding whether to prescribe rivaroxaban to patients at increased risk of bleeding, the risk of thrombotic events should be weighed against the risk of bleeding.
- Promptly evaluate any signs or symptoms of blood loss and consider the need for blood replacement.
- Discontinue rivaroxaban in patients with active pathological hemorrhage. The terminal elimination half-life of rivaroxaban is 5 to 9 hours in healthy subjects aged 20 to 45 years.
- Concomitant use of other drugs that impair hemostasis increases the risk of bleeding. These include aspirin, P2Y12 platelet inhibitors, other antithrombotic agents, fibrinolytic therapy, and non-steroidal anti-inflammatory drugs (NSAIDs).
- Concomitant use of drugs that are combined P-gp and CYP3A4 inhibitors (e.g., ketoconazole and ritonavir) increases rivaroxaban exposure and may increase bleeding risk.
Reversal of Anticoagulant Effect=
- A specific antidote for rivaroxaban is not available. Because of high plasma protein binding, rivaroxaban is not expected to be dialyzable.
- Protamine sulfate and vitamin K are not expected to affect the anticoagulant activity of rivaroxaban.
- Partial reversal of prothrombin time prolongation has been seen after administration of prothrombin complex concentrates (PCCs) in healthy volunteers.
- The use of other procoagulant reversal agents like activated prothrombin complex concentrate (APCC) or recombinant factor VIIa (rFVIIa) has not been evaluated.
Spinal/Epidural Anesthesia or Puncture
- When neuraxial anesthesia (spinal/epidural anesthesia) or spinal puncture is employed, patients treated with anticoagulant agents for prevention of thromboembolic complications are at risk of developing an epidural or spinal hematoma which can result in long-term or permanent paralysis.
- An epidural catheter should not be removed earlier than 18 hours after the last administration of rivaroxaban.
- The next rivaroxaban dose is not to be administered earlier than 6 hours after the removal of the catheter.
- If traumatic puncture occurs, the administration of rivaroxaban is to be delayed for 24 hours.
Use in Patients with Renal Impairment
Nonvalvular Atrial Fibrillation
- Avoid the use of rivaroxaban in patients with CrCl <15 mL/min since drug exposure is increased.
- Periodically assess renal function as clinically indicated (i.e., more frequently in situations in which renal function may decline) and adjust therapy accordingly.
- Discontinue rivaroxaban in patients who develop acute renal failure while on rivaroxaban.
Treatment of Deep Vein Thrombosis (DVT), Pulmonary Embolism (PE), and Reduction in the Risk of Recurrence of DVT and of PE
- Avoid the use of rivaroxaban in patients with CrCl <30 mL/min due to an expected increase in rivaroxaban exposure and pharmacodynamic effects in this patient population.
Prophylaxis of Deep Vein Thrombosis Following Hip or Knee Replacement Surgery
- Avoid the use of rivaroxaban in patients with CrCl <30 mL/min due to an expected increase in rivaroxaban exposure and pharmacodynamic effects in this patient population.
- Observe closely and promptly evaluate any signs or symptoms of blood loss in patients with CrCl 30 to 50 mL/min.
- Patients who develop acute renal failure while on rivaroxaban should discontinue the treatment.
Use in Patients with Hepatic Impairment
- No clinical data are available for patients with severe hepatic impairment.
- Avoid use of rivaroxaban in patients with moderate (Child-Pugh B) and severe (Child-Pugh C) hepatic impairment or with any hepatic disease associated with coagulopathy since drug exposure and bleeding risk may be increased.
Use with P-gp and Strong CYP3A4 Inhibitors or Inducers
- Avoid concomitant use of rivaroxaban with combined P-gp and strong CYP3A4 inhibitors (e.g., ketoconazole, itraconazole, lopinavir/ritonavir, ritonavir, indinavir, and conivaptan).
- Avoid concomitant use of rivaroxaban with drugs that are combined P-gp and strong CYP3A4 inducers (e.g., carbamazepine, phenytoin, rifampin, St. John's wort).
Risk of Pregnancy-Related Hemorrhage
- In pregnant women, rivaroxaban should be used only if the potential benefit justifies the potential risk to the mother and fetus. rivaroxaban dosing in pregnancy has not been studied.
- The anticoagulant effect of rivaroxaban cannot be monitored with standard laboratory testing nor readily reversed.
- Promptly evaluate any signs or symptoms suggesting blood loss (e.g., a drop in hemoglobin and/or hematocrit, hypotension, or fetal distress).
Patients with Prosthetic Heart Valves
- The safety and efficacy of rivaroxaban have not been studied in patients with prosthetic heart valves. Therefore, use of rivaroxaban is not recommended in these patients.
Acute PE in Hemodynamically Unstable Patients or Patients Who Require Thrombolysis or Pulmonary Embolectomy
- Initiation of rivaroxaban is not recommended acutely as an alternative to unfractionated heparin in patients with pulmonary embolism who present with hemodynamic instability or who may receive thrombolysis or pulmonary embolectomy
|clinicalTrials=During clinical development for the approved indications, 16326 patients were exposed to rivaroxaban. These included 7111 patients who received rivaroxaban 15 mg or 20 mg orally once daily for a mean of 19 months (5558 for 12 months and 2512 for 24 months) to reduce the risk of stroke and systemic embolism in nonvalvular atrial fibrillation (ROCKET AF); 4728 patients who received either rivaroxaban 15 mg orally twice daily for three weeks followed by 20 mg orally once daily (EINSTEIN DVT, EINSTEIN PE) or 20 mg orally once daily (EINSTEIN Extension) to treat DVT, PE, and to reduce the risk of recurrence of DVT and of PE; and 4487 patients who received rivaroxaban 10 mg orally once daily for prophylaxis of DVT following hip or knee replacement surgery (RECORD 1–3).
Hemorrhage
The most common adverse reactions with rivaroxaban were bleeding complications.
Nonvalvular Atrial Fibrillation
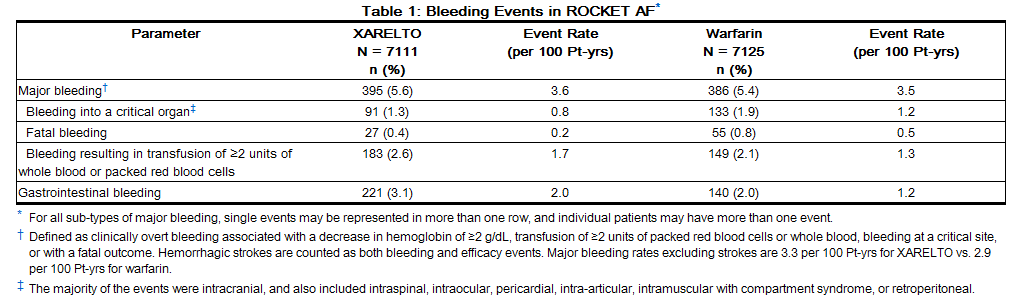
In the ROCKET AF trial, the most frequent adverse reactions associated with permanent drug discontinuation were bleeding events, with incidence rates of 4.3% for rivaroxaban vs. 3.1% for warfarin. The incidence of discontinuations for non-bleeding adverse events was similar in both treatment groups.
Table 1 shows the number of patients experiencing various types of bleeding events in the ROCKET AF trial.
Treatment of Deep Vein Thrombosis (DVT), Pulmonary Embolism (PE), and to Reduce the Risk of Recurrence of DVT and of PE
EINSTEIN DVT and EINSTEIN PE Studies
In the pooled analysis of the EINSTEIN DVT and EINSTEIN PE clinical studies, the most frequent adverse reactions leading to permanent drug discontinuation were bleeding events, with rivaroxaban vs. enoxaparin/Vitamin K antagonist (VKA) incidence rates of 1.7% vs. 1.5%, respectively. The mean duration of treatment was 208 days for rivaroxaban-treated patients and 204 days for enoxaparin/VKA-treated patients.
Table 2 shows the number of patients experiencing major bleeding events in the pooled analysis of the EINSTEIN DVT and EINSTEIN PE studies.

EINSTEIN Extension Study
In the EINSTEIN Extension clinical study, the most frequent adverse reactions associated with permanent drug discontinuation were bleeding events, with incidence rates of 1.8% for rivaroxaban vs. 0.2% for placebo treatment groups. The mean duration of treatment was 190 days for both rivaroxaban and placebo treatment groups.
Table 3 shows the number of patients experiencing bleeding events in the EINSTEIN Extension study.

Prophylaxis of Deep Vein Thrombosis Following Hip or Knee Replacement Surgery
In the RECORD clinical trials, the overall incidence rate of adverse reactions leading to permanent treatment discontinuation was 3.7% with rivaroxaban.
The rates of major bleeding events and any bleeding events observed in patients in the RECORD clinical trials are shown in Table 4.

Following rivaroxaban treatment, the majority of major bleeding complications (≥60%) occurred during the first week after surgery.
Other Adverse Reactions
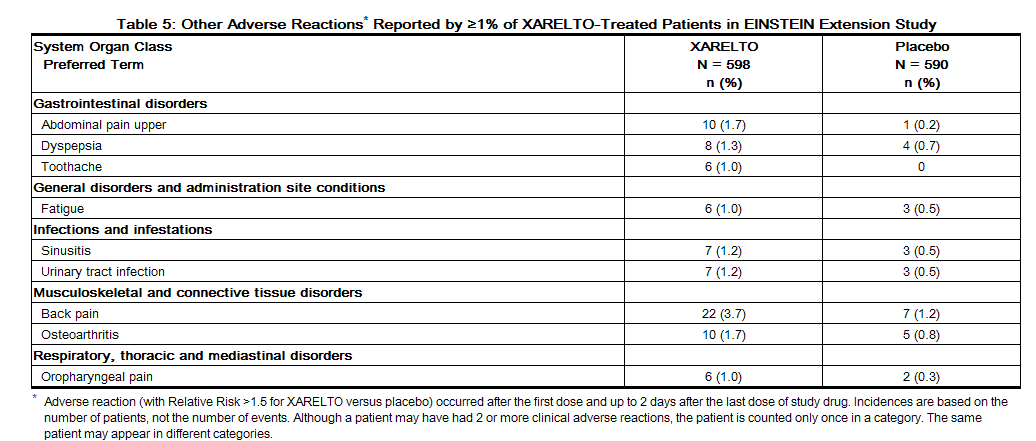
Non-hemorrhagic adverse reactions reported in ≥1% of rivaroxaban-treated patients in the EINSTEIN Extension study are shown in Table 5.
Non-hemorrhagic adverse reactions reported in ≥1% of rivaroxaban-treated patients in RECORD 1–3 studies are shown in Table 6.

Other clinical trial experience: In an investigational study of acute medically ill patients being treated with rivaroxaban 10 mg tablets, cases of pulmonary hemorrhage and pulmonary hemorrhage with bronchiectasis were observed. |postmarketing=The following adverse reactions have been identified during post-approval use of rivaroxaban. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Blood and lymphatic system disorders
Gastrointestinal disorders
Hepatobiliary disorders
- Jaundice
- Cholestasis
- Cytolytic hepatitis
Immune system disorders
Nervous system disorders
Skin and subcutaneous tissue disorders
|drugInteractions=Rivaroxaban is a substrate of CYP3A4/5, CYP2J2, and the P-gp and ATP-binding cassette G2 (ABCG2) transporters. Inhibitors and inducers of these CYP450 enzymes or transporters (e.g., P-gp) may result in changes in rivaroxaban exposure.
Drugs that Inhibit Cytochrome P450 3A4 Enzymes and Drug Transport Systems
- In drug interaction studies evaluating the concomitant use with drugs that are combined P-gp and CYP3A4 inhibitors (ketoconazole, ritonavir, clarithromycin, erythromycin and fluconazole), increases in rivaroxaban exposure and pharmacodynamic effects (i.e., factor Xa inhibition and PT prolongation) were observed.
- The increases in exposure ranged from 30% to 160%. Significant increases in rivaroxaban exposure may increase bleeding risk.
- When data suggest a change in exposure is unlikely to affect bleeding risk (e.g., clarithromycin, erythromycin), no precautions are necessary during coadministration with drugs that are combined P-gp and CYP3A4 inhibitors.
- Avoid concomitant administration of rivaroxaban with combined P-gp and strong CYP3A4 inhibitors.
Drugs that Induce Cytochrome P450 3A4 Enzymes and Drug Transport Systems
- Results from drug interaction studies and population PK analyses from clinical studies indicate coadministration of rivaroxaban with a combined P-gp and strong CYP3A4 inducer (e.g., rifampicin, phenytoin) decreased rivaroxaban exposure by up to 50%.
- Similar decreases in pharmacodynamic effects were also observed.
- These decreases in exposure to rivaroxaban may decrease efficacy.
- Avoid concomitant use of rivaroxaban with drugs that are combined P-gp and strong CYP3A4 inducers (e.g., carbamazepine, phenytoin, rifampin, St. John's wort).
Anticoagulants and NSAIDs/Aspirin
- Single doses of enoxaparin and rivaroxaban given concomitantly resulted in an additive effect on anti-factor Xa activity.
- Single doses of warfarin and rivaroxaban resulted in an additive effect on factor Xa (FXa) inhibition and PT.
- Concomitant aspirin use has been identified as an independent risk factor for major bleeding in efficacy trials.
- NSAIDs are known to increase bleeding, and bleeding risk may be increased when NSAIDs are used concomitantly with rivaroxaban.
- Coadministration of the platelet aggregation inhibitor clopidogrel and rivaroxaban resulted in an increase in bleeding time for some subjects.
- Avoid concurrent use of rivaroxaban with other anticoagulants due to increased bleeding risk unless benefit outweighs risk.
- Promptly evaluate any signs or symptoms of blood loss if patients are treated concomitantly with aspirin, other platelet aggregation inhibitors, or NSAIDs.
Drug-Disease Interactions with Drugs that Inhibit Cytochrome P450 3A4 Enzymes and Drug Transport Systems
- Results from a pharmacokinetic trial with erythromycin indicated that patients with renal impairment coadministered rivaroxaban with drugs classified as combined P-gp and moderate CYP3A4 inhibitors (e.g., diltiazem, verapamil, dronedarone, and erythromycin) have increased exposure compared with patients with normal renal function and no inhibitor use.
- Significant increases in rivaroxaban exposure may increase bleeding risk.
- While increases in rivaroxaban exposure can be expected under such conditions, results from an analysis in the ROCKET AF trial, which allowed concomitant use with combined P-gp and weak or moderate CYP3A4 inhibitors (e.g., amiodarone, diltiazem, verapamil, chloramphenicol, cimetidine, and erythromycin), did not show an increase in bleeding in patients with CrCl 30 to <50 mL/min [Hazard Ratio (95% CI): 1.05 (0.77, 1.42)].
- Rivaroxaban should not be used in patients with CrCl 15 to 80 mL/min who are receiving concomitant combined P-gp and moderate CYP3A4 inhibitors unless the potential benefit justifies the potential risk
|FDAPregCat=C |useInPregnancyFDA=* There are no adequate or well-controlled studies of rivaroxaban in pregnant women, and dosing for pregnant women has not been established.
- Use rivaroxaban with caution in pregnant patients because of the potential for pregnancy related hemorrhage and/or emergent delivery with an anticoagulant that is not readily reversible.
- The anticoagulant effect of rivaroxaban cannot be reliably monitored with standard laboratory testing.
- Animal reproduction studies showed no increased risk of structural malformations, but increased post-implantation pregnancy loss occurred in rabbits.
- Rivaroxaban should be used during pregnancy only if the potential benefit justifies the potential risk to mother and fetus.
- Rivaroxaban crosses the placenta in animals. Animal reproduction studies have shown pronounced maternal hemorrhagic complications in rats and an increased incidence of post‑implantation pregnancy loss in rabbits.
- Rivaroxaban increased fetal toxicity (increased resorptions, decreased number of live fetuses, and decreased fetal body weight) when pregnant rabbits were given oral doses of ≥10 mg/kg rivaroxaban during the period of organogenesis.
- This dose corresponds to about 4 times the human exposure of unbound drug, based on AUC comparisons at the highest recommended human dose of 20 mg/day.
- Fetal body weights decreased when pregnant rats were given oral doses of 120 mg/kg. This dose corresponds to about 14 times the human exposure of unbound drug.
|useInLaborDelivery=*Safety and effectiveness of rivaroxaban during labor and delivery have not been studied in clinical trials. However, in animal studies maternal bleeding and maternal and fetal death occurred at the rivaroxaban dose of 40 mg/kg (about 6 times maximum human exposure of the unbound drug at the human dose of 20 mg/day). |useInNursing=*It is not known if rivaroxaban is excreted in human milk. Rivaroxaban and/or its metabolites were excreted into the milk of rats.
- Because many drugs are excreted in human milk and because of the potential for serious adverse reactions in nursing infants from rivaroxaban, a decision should be made whether to discontinue nursing or discontinue rovaroxaban, taking into account the importance of the drug to the mother.
|useInPed=*Safety and effectiveness in pediatric patients have not been established. |useInGeri=* Of the total number of patients in the RECORD 1–3 clinical studies evaluating rivaroxaban , about 54% were 65 years and over, while about 15% were >75 years. In ROCKET AF, approximately 77% were 65 years and over and about 38% were >75 years.
- In the EINSTEIN DVT, PE and Extension clinical studies approximately 37% were 65 years and over and about 16% were >75 years.
- In clinical trials the efficacy of rivaroxaban in the elderly (65 years or older) was similar to that seen in patients younger than 65 years.
- Both thrombotic and bleeding event rates were higher in these older patients, but the risk-benefit profile was favorable in all age groups
|useInRenalImpair=====Nonvalvular Atrial Fibrillation ====
- In the ROCKET AF trial, patients with CrCl 30 to 50 mL/min were administered rivaroxaban 15 mg once daily resulting in serum concentrations of rivaroxaban and clinical outcomes similar to those in patients with better renal function administered rivaroxaban 20 mg once daily.
- Patients with CrCl 15 to 30 mL/min were not studied, but administration of rivaroxaban 15 mg once daily is also expected to result in serum concentrations of rivaroxaban similar to those in patients with normal renal function.
Treatment of DVT and/or PE, and Reduction in the Risk of Recurrence of DVT and of PE
- In the EINSTEIN trials, patients with CrCl values <30 mL/min at screening were excluded from the studies.
- Avoid the use of rivaroxaban in patients with CrCl <30 mL/min.
Prophylaxis of DVT Following Hip or Knee Replacement Surgery
- The combined analysis of the RECORD 1–3 clinical efficacy studies did not show an increase in bleeding risk for patients with CrCl 30 to 50 mL/min and reported a possible increase in total venous thromboemboli in this population.
- Observe closely and promptly evaluate any signs or symptoms of blood loss in patients with CrCl 30 to 50 mL/min. Avoid the use of rivaroxaban in patients with CrCl <30 mL/min.
|useInHepaticImpair=*In a pharmacokinetic study, compared to healthy subjects with normal liver function, AUC increases of 127% were observed in subjects with moderate hepatic impairment (Child-Pugh B).
- The safety or PK of rivaroxaban in patients with severe hepatic impairment (Child-Pugh C) has not been evaluated.
- Avoid the use of rivaroxaban in patients with moderate (Child-Pugh B) and severe (Child-Pugh C) hepatic impairment or with any hepatic disease associated with coagulopathy.
|useInReproPotential=*Females of reproductive potential requiring anticoagulation should discuss pregnancy planning with their physician. |othersTitle=Others |administration=Oral |monitoring=====Important Food Effect Information====
- The 15 mg and 20 mg rivaroxaban tablets should be taken with food, while the 10 mg tablet can be taken with or without food [see Clinical Pharmacology (12.3)].
- In the nonvalvular atrial fibrillation efficacy study rivaroxaban was taken with the evening meal.
Switching to and from rivaroxaban
- Switching from warfarin to rivaroxaban - When switching patients from warfarin to rivaroxaban, discontinue warfarin and start rivaroxaban as soon as the International Normalized Ratio (INR) is below 3.0 to avoid periods of inadequate anticoagulation.
- Switching from rivaroxaban to warfarin - No clinical trial data are available to guide converting patients from rivaroxaban to warfarin. rivaroxaban affects INR, so INR measurements made during coadministration with warfarin may not be useful for determining the appropriate dose of warfarin. One approach is to discontinue rivaroxaban and begin both a parenteral anticoagulant and warfarin at the time the next dose of rivaroxaban would have been taken.
- Switching from rivaroxaban to anticoagulants other than warfarin - For patients currently taking rivaroxaban and transitioning to an anticoagulant with rapid onset, discontinue rivaroxaban and give the first dose of the other anticoagulant (oral or parenteral) at the time that the next rivaroxaban dose would have been taken.
- Switching from anticoagulants other than warfarin to rivaroxaban - For patients currently receiving an anticoagulant other than warfarin, start rivaroxaban 0 to 2 hours prior to the next scheduled evening administration of the drug (e.g., low molecular weight heparin or non-warfarin oral anticoagulant) and omit administration of the other anticoagulant. For unfractionated heparin being administered by continuous infusion, stop the infusion and start rivaroxaban at the same time.
Discontinuation for Surgery and other Interventions
- If anticoagulation must be discontinued to reduce the risk of bleeding with surgical or other procedures, rivaroxaban should be stopped at least 24 hours before the procedure to reduce the risk of bleeding.
- In deciding whether a procedure should be delayed until 24 hours after the last dose of rivaroxaban, the increased risk of bleeding should be weighed against the urgency of intervention.
- Rivaroxaban should be restarted after the surgical or other procedures as soon as adequate hemostasis has been established, noting that the time to onset of therapeutic effect is short.
- If oral medication cannot be taken during or after surgical intervention, consider administering a parenteral anticoagulant.
Missed Dose
- If a dose of rivaroxaban is not taken at the scheduled time, administer the dose as soon as possible on the same day as follows:
- For patients receiving 15 mg twice daily: The patient should take rivaroxaban immediately to ensure intake of 30 mg rivaroxaban per day. In this particular instance, two 15 mg tablets may be taken at once. The patient should continue with the regular 15 mg twice daily intake as recommended on the following day.
- For patients receiving 20 mg, 15 mg or 10 mg once daily: The patient should take the missed rivaroxaban dose immediately.
Administration Options
- For patients who are unable to swallow whole tablets, 15 mg or 20 mg rivaroxaban tablets may be crushed and mixed with applesauce immediately prior to use and administered orally.
- After the administration of a crushed rivaroxaban 15 mg or 20 mg tablet, the dose should be immediately followed by food.
Administration via nasogastric (NG) tube or gastric feeding tube
- After confirming gastric placement of the tube, 15 mg or 20 mg rivaroxaban tablets may be crushed and suspended in 50 mL of water and administered via an NG tube or gastric feeding tube.
- Since rivaroxaban absorption is dependent on the site of drug release, avoid administration of rivaroxaban distal to the stomach which can result in reduced absorption and thereby, reduced drug exposure.
- After the administration of a crushed rivaroxaban 15 mg or 20 mg tablet, the dose should then be immediately followed by enteral feeding.
- Crushed 15 mg or 20 mg rivaroxaban tablets are stable in water and in applesauce for up to 4 hours.
- An in vitro compatibility study indicated that there is no adsorption of rivaroxaban from a water suspension of a crushed rivaroxaban tablet to PVC or silicone nasogastric (NG) tubing
|IVCompat====Solution===
Compatible
- Solution 1
- Solution 2
- Solution 3
Not Tested
- Solution 1
- Solution 2
- Solution 3
Variable
- Solution 1
- Solution 2
- Solution 3
Incompatible
- Solution 1
- Solution 2
- Solution 3
Y-Site
Compatible
- Solution 1
- Solution 2
- Solution 3
Not Tested
- Solution 1
- Solution 2
- Solution 3
Variable
- Solution 1
- Solution 2
- Solution 3
Incompatible
- Solution 1
- Solution 2
- Solution 3
Admixture
Compatible
- Solution 1
- Solution 2
- Solution 3
Not Tested
- Solution 1
- Solution 2
- Solution 3
Variable
- Solution 1
- Solution 2
- Solution 3
Incompatible
- Solution 1
- Solution 2
- Solution 3
Syringe
Compatible
- Solution 1
- Solution 2
- Solution 3
Not Tested
- Solution 1
- Solution 2
- Solution 3
Variable
- Solution 1
- Solution 2
- Solution 3
Incompatible
- Solution 1
- Solution 2
- Solution 3
TPN/TNA
Compatible
- Solution 1
- Solution 2
- Solution 3
Not Tested
- Solution 1
- Solution 2
- Solution 3
Variable
- Solution 1
- Solution 2
- Solution 3
Incompatible
- Solution 1
- Solution 2
- Solution 3
|overdose=*Overdose of rivaroxaban may lead to hemorrhage.
- Discontinue rivaroxaban and initiate appropriate therapy if bleeding complications associated with overdosage occur.
- A specific antidote for rivaroxaban is not available.
- Rivaroxaban systemic exposure is not further increased at single doses >50 mg due to limited absorption.
- The use of activated charcoal to reduce absorption in case of rivaroxaban overdose may be considered.
- Due to the high plasma protein binding, rivaroxaban is not expected to be dialyzable.
- Partial reversal of laboratory anticoagulation parameters may be achieved with use of plasma products.
|drugBox={{Drugbox2
| Verifiedfields = changed
| Watchedfields = changed
| verifiedrevid = 459434616
| IUPAC_name = (S)-5-chloro-N-{[2-oxo-3-[4-(3-oxomorpholin-4-yl)
phenyl]oxazolidin-5-yl]methyl} thiophene-2-carboxamide
| image = Rivaroxaban structure.png
| width = 250
| image2 = Rivaroxaban ball-and-stick.png
| tradename = Xarelto
| Drugs.com = Micromedex Detailed Consumer Information
| licence_EU = Xarelto
| licence_US = Rivaroxaban
| pregnancy_US = C
| legal_UK = POM
| legal_US = Rx-only
| legal_status =
| routes_of_administration = oral
| bioavailability = 80% to 100%; Cmax = 2–4 hours (10 mg oral)[1] | metabolism = CYP3A4 , CYP2J2 and CYP-independent mechanisms[1] | elimination_half-life = 10 mg oral 7–11 hours[1] | excretion = 2/3 metabolized in liver and 1/3 eliminated unchanged[1]
| CAS_number_Ref =
| CAS_number = 366789-02-8
| ATC_prefix = B01
| ATC_suffix = AF01
| PubChem = 6433119
| ChEBI_Ref =
| ChEBI = 68579
| DrugBank_Ref =
| DrugBank = DB06228
| ChemSpiderID_Ref =
| ChemSpiderID = 8051086
| UNII_Ref =
| UNII = 9NDF7JZ4M3
| ChEMBL_Ref =
| ChEMBL = 198362
| PDB_ligand = RIV
| C=19 | H=18 | Cl=1 | N=3 | O=5 | S=1
| molecular_weight = 435.882 g/mol
| smiles = c1cc(ccc1N2CCOCC2=O)N3C[C@@H](OC3=O)CNC(=O)c4ccc(s4)Cl
| InChI = 1/C19H18ClN3O5S/c20-16-6-5-15(29-16)18(25)21-9-14-10-23(19(26)28-14)13-3-1-12(2-4-13)22-7-8-27-11-17(22)24/h1-6,14H,7-11H2,(H,21,25)/t14-/m0/s1
| InChIKey = KGFYHTZWPPHNLQ-AWEZNQCLBK
| StdInChI_Ref =
| StdInChI = 1S/C19H18ClN3O5S/c20-16-6-5-15(29-16)18(25)21-9-14-10-23(19(26)28-14)13-3-1-12(2-4-13)22-7-8-27-11-17(22)24/h1-6,14H,7-11H2,(H,21,25)/t14-/m0/s1
| StdInChIKey_Ref =
| StdInChIKey = KGFYHTZWPPHNLQ-AWEZNQCLSA-N
}}
|mechAction=(Description)
|structure=(Description with picture)
|PD=(Description)
|PK=(Description)
|nonClinToxic=(Description)
|clinicalStudies======Condition 1=====
(Description)
Condition 2
(Description)
Condition 3
(Description) |howSupplied=(Description) |fdaPatientInfo=(Patient Counseling Information) |alcohol=Alcohol-Rivaroxaban interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication. |lookAlike=* (Paired Confused Name 1a) — (Paired Confused Name 1b)
- (Paired Confused Name 2a) — (Paired Confused Name 2b)
- (Paired Confused Name 3a) — (Paired Confused Name 3b)
}}