Rivaroxaban: Difference between revisions
No edit summary |
No edit summary |
||
| Line 473: | Line 473: | ||
*Partial reversal of laboratory [[anticoagulation]] parameters may be achieved with use of plasma products. | *Partial reversal of laboratory [[anticoagulation]] parameters may be achieved with use of plasma products. | ||
|drugBox={{Drugbox2 | |drugBox={{Drugbox2 | ||
| verifiedrevid = | | Verifiedfields = changed | ||
| IUPAC_name = | | verifiedrevid = 464383832 | ||
| image = | | IUPAC_name = (3''R'',5''S'',6''E'')-7-[4-(4-fluorophenyl)-2-(''N''-methylmethanesulfonamido)-6-(propan-2-yl)pyrimidin-5-yl]-3,5-dihydroxyhept-6-enoic acid | ||
| | | image = Rosuvastatin2DCSD.svg | ||
| width = 250 | |||
| image2 = Rosuvastatin3Dan.gif | |||
| width2 = 250 | |||
<!--Clinical data--> | <!--Clinical data--> | ||
| tradename = | | tradename = Crestor, R2 | ||
| | | Drugs.com = {{drugs.com|monograph|crestor}} | ||
| | | MedlinePlus = a603033 | ||
| pregnancy_AU = | | pregnancy_AU = D | ||
| pregnancy_US = | | pregnancy_US = X | ||
| legal_status = | | pregnancy_category = | ||
| routes_of_administration = | | legal_AU = S4 | ||
| legal_UK = POM | |||
| legal_US = Rx-only | |||
| legal_status = | |||
| routes_of_administration = oral | |||
<!--Pharmacokinetic data--> | <!--Pharmacokinetic data--> | ||
| bioavailability = | | bioavailability = 20%<ref name=PK>{{cite journal|last=Aggarwal|first=RK|coauthors=Showkathali, R|title=Rosuvastatin calcium in acute coronary syndromes|journal=Expert Opinion on Pharmacotherapy|date=June 2013|volume=14|issue=9|pages=1215–1227|doi=10.1517/14656566.2013.789860|pmid=23574635}}</ref> | ||
| metabolism = | | protein_bound = 88%<ref name = PK/> | ||
| elimination_half-life = | | metabolism = [[Liver]] ([[CYP2C9]] and [[CYP2C19]]-mediated; only minimally (~10%) metabolised)<ref name = PK/> | ||
| excretion = | | elimination_half-life = 19 hours<ref name = PK/> | ||
| excretion = Faeces<ref name = PK/> | |||
<!--Identifiers--> | <!--Identifiers--> | ||
| CAS_number_Ref = | | CASNo_Ref = {{cascite|correct|CAS}} | ||
| CAS_number = | | CAS_number_Ref = {{cascite|correct|??}} | ||
| ATC_prefix = | | CAS_number = 287714-41-4 | ||
| ATC_suffix = | | ATC_prefix = C10 | ||
| PubChem = | | ATC_suffix = AA07 | ||
| | | PubChem = 446157 | ||
| | | DrugBank_Ref = {{drugbankcite|correct|drugbank}} | ||
| DrugBank = | | DrugBank = DB01098 | ||
| ChemSpiderID_Ref = | | ChemSpiderID_Ref = {{chemspidercite|changed|chemspider}} | ||
| ChemSpiderID = | | ChemSpiderID = 393589 | ||
| UNII_Ref = | | UNII_Ref = {{fdacite|correct|FDA}} | ||
| UNII = | | UNII = 413KH5ZJ73 | ||
| KEGG_Ref = | | KEGG_Ref = {{keggcite|changed|kegg}} | ||
| KEGG = | | KEGG = D01915 | ||
| ChEBI_Ref = | | ChEBI_Ref = {{ebicite|changed|EBI}} | ||
| ChEBI = | | ChEBI = 38545 | ||
| ChEMBL_Ref = | | ChEMBL_Ref = {{ebicite|changed|EBI}} | ||
| ChEMBL = | | ChEMBL = 1496 | ||
| PDB_ligand = FBI | |||
<!--Chemical data--> | <!--Chemical data--> | ||
| C= | H= | N= | O= | | C=22 | H=28 | F=1 | N=3 | O=6 | S=1 | ||
| molecular_weight = | | molecular_weight = 481.539 | ||
| smiles = | | smiles = O=S(=O)(N(c1nc(c(c(n1)C(C)C)/C=C/[C@@H](O)C[C@@H](O)CC(=O)O)c2ccc(F)cc2)C)C | ||
| InChI = | | InChI = 1/C22H28FN3O6S/c1-13(2)20-18(10-9-16(27)11-17(28)12-19(29)30)21(14-5-7-15(23)8-6-14)25-22(24-20)26(3)33(4,31)32/h5-10,13,16-17,27-28H,11-12H2,1-4H3,(H,29,30)/b10-9+/t16-,17-/m1/s1 | ||
| InChIKey = | | InChIKey = BPRHUIZQVSMCRT-VEUZHWNKBK | ||
| StdInChI_Ref = | | StdInChI_Ref = {{stdinchicite|changed|chemspider}} | ||
| StdInChI = | | StdInChI = 1S/C22H28FN3O6S/c1-13(2)20-18(10-9-16(27)11-17(28)12-19(29)30)21(14-5-7-15(23)8-6-14)25-22(24-20)26(3)33(4,31)32/h5-10,13,16-17,27-28H,11-12H2,1-4H3,(H,29,30)/b10-9+/t16-,17-/m1/s1 | ||
| StdInChIKey_Ref = | | StdInChIKey_Ref = {{stdinchicite|changed|chemspider}} | ||
| StdInChIKey = | | StdInChIKey = BPRHUIZQVSMCRT-VEUZHWNKSA-N | ||
}} | }} | ||
|mechAction=(Description) | |mechAction=(Description) | ||
Revision as of 18:59, 2 July 2014
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Alejandro Lemor, M.D. [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Black Box Warning
|
(A) PREMATURE DISCONTINUATION OF XARELTO INCREASES THE RISK OF THROMBOTIC EVENTS,
(B) SPINAL/EPIDURAL HEMATOMA See full prescribing information for complete Boxed Warning.
PREMATURE DISCONTINUATION OF RIVAROXABAN INCREASES THE RISK OF THROMBOTIC EVENTS: Premature discontinuation of any oral anticoagulant, including rivaroxaban, increases the risk of thrombotic events. If anticoagulation with rivaroxaban is discontinued for a reason other than pathological bleeding or completion of a course of therapy, consider coverage with another anticoagulant.
Monitor patients frequently for signs and symptoms of neurological impairment. If neurological compromise is noted, urgent treatment is necessary. Consider the benefits and risks before neuraxial intervention in patients anticoagulated or to be anticoagulated for thromboprophylaxis |
Overview
Rivaroxaban is a Factor Xa inhibitor that is FDA approved for the {{{indicationType}}} of reduction in the risk of stroke and systemic embolism in patients with nonvalvular atrial fibrillation, treatment of deep vein thrombosis (DVT), pulmonary embolism (PE), reduction in the risk of recurrence of DVT and of PE, and for the prophylaxis of DVT, which may lead to PE in patients undergoing knee or hip replacement surgery.. There is a Black Box Warning for this drug as shown here. Common adverse reactions include bleeding.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Reduction of Risk of Stroke and Systemic Embolism in Nonvalvular Atrial Fibrillation
- Rivaroxaban is indicated to reduce the risk of stroke and systemic embolism in patients with nonvalvular atrial fibrillation.
- There are limited data on the relative effectiveness of Rivaroxaban and warfarin in reducing the risk of stroke and systemic embolism when warfarin therapy is well-controlled.
Treatment of Deep Vein Thrombosis
- Rivaroxaban is indicated for the treatment of deep vein thrombosis (DVT).
Treatment of Pulmonary Embolism
- Rivaroxaban is indicated for the treatment of pulmonary embolism (PE).
Reduction in the Risk of Recurrence of Deep Vein Thrombosis and of Pulmonary Embolism
- Rivaroxaban is indicated for the reduction in the risk of recurrence of deep vein thrombosis and of pulmonary embolism following initial 6 months treatment for DVT and/or PE.
Prophylaxis of Deep Vein Thrombosis Following Hip or Knee Replacement Surgery
- Rivaroxaban is indicated for the prophylaxis of DVT, which may lead to PE in patients undergoing knee or hip replacement surgery
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
Condition 1
- Developed by: (Organisation)
- Class of Recommendation: (Class) (Link)
- Strength of Evidence: (Category A/B/C) (Link)
- Dosing Information/Recommendation
- (Dosage)
Condition 2
- Developed by: (Organisation)
- Class of Recommendation: (Class) (Link)
- Strength of Evidence: (Category A/B/C) (Link)
- Dosing Information/Recommendation
- (Dosage)
Non–Guideline-Supported Use
Condition 1
- Dosing Information
- (Dosage)
Condition 2
- Dosing Information
- (Dosage)
Condition 3
- Dosing Information
- (Dosage)
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
Condition 1
- Dosing Information
- (Dosage)
Condition 2
- Dosing Information
- (Dosage)
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
Condition 1
- Developed by: (Organisation)
- Class of Recommendation: (Class) (Link)
- Strength of Evidence: (Category A/B/C) (Link)
- Dosing Information/Recommendation
- (Dosage)
Condition 2
- Developed by: (Organisation)
- Class of Recommendation: (Class) (Link)
- Strength of Evidence: (Category A/B/C) (Link)
- Dosing Information/Recommendation
- (Dosage)
Non–Guideline-Supported Use
Condition 1
- Dosing Information
- (Dosage)
Condition 2
- Dosing Information
- (Dosage)
Condition 3
- Dosing Information
- (Dosage)
Contraindications
- Active pathological bleeding
- Severe hypersensitivity reaction to rivaroxaban
Warnings
|
(A) PREMATURE DISCONTINUATION OF XARELTO INCREASES THE RISK OF THROMBOTIC EVENTS,
(B) SPINAL/EPIDURAL HEMATOMA See full prescribing information for complete Boxed Warning.
PREMATURE DISCONTINUATION OF RIVAROXABAN INCREASES THE RISK OF THROMBOTIC EVENTS: Premature discontinuation of any oral anticoagulant, including rivaroxaban, increases the risk of thrombotic events. If anticoagulation with rivaroxaban is discontinued for a reason other than pathological bleeding or completion of a course of therapy, consider coverage with another anticoagulant.
Monitor patients frequently for signs and symptoms of neurological impairment. If neurological compromise is noted, urgent treatment is necessary. Consider the benefits and risks before neuraxial intervention in patients anticoagulated or to be anticoagulated for thromboprophylaxis |
Increased Risk of Thrombotic Events after Premature Discontinuation
- Premature discontinuation of any oral anticoagulant, including rivaroxaban, in the absence of adequate alternative anticoagulation increases the risk of thrombotic events.
- An increased rate of stroke was observed during the transition from rivaroxaban to warfarin in clinical trials in atrial fibrillation patients.
- If rivaroxaban is discontinued for a reason other than pathological bleeding or completion of a course of therapy, consider coverage with another anticoagulant.
Risk of Bleeding
- Rivaroxaban increases the risk of bleeding and can cause serious or fatal bleeding. In deciding whether to prescribe rivaroxaban to patients at increased risk of bleeding, the risk of thrombotic events should be weighed against the risk of bleeding.
- Promptly evaluate any signs or symptoms of blood loss and consider the need for blood replacement.
- Discontinue rivaroxaban in patients with active pathological hemorrhage. The terminal elimination half-life of rivaroxaban is 5 to 9 hours in healthy subjects aged 20 to 45 years.
- Concomitant use of other drugs that impair hemostasis increases the risk of bleeding. These include aspirin, P2Y12 platelet inhibitors, other antithrombotic agents, fibrinolytic therapy, and non-steroidal anti-inflammatory drugs (NSAIDs).
- Concomitant use of drugs that are combined P-gp and CYP3A4 inhibitors (e.g., ketoconazole and ritonavir) increases rivaroxaban exposure and may increase bleeding risk.
Reversal of Anticoagulant Effect=
- A specific antidote for rivaroxaban is not available. Because of high plasma protein binding, rivaroxaban is not expected to be dialyzable.
- Protamine sulfate and vitamin K are not expected to affect the anticoagulant activity of rivaroxaban.
- Partial reversal of prothrombin time prolongation has been seen after administration of prothrombin complex concentrates (PCCs) in healthy volunteers.
- The use of other procoagulant reversal agents like activated prothrombin complex concentrate (APCC) or recombinant factor VIIa (rFVIIa) has not been evaluated.
Spinal/Epidural Anesthesia or Puncture
- When neuraxial anesthesia (spinal/epidural anesthesia) or spinal puncture is employed, patients treated with anticoagulant agents for prevention of thromboembolic complications are at risk of developing an epidural or spinal hematoma which can result in long-term or permanent paralysis.
- An epidural catheter should not be removed earlier than 18 hours after the last administration of rivaroxaban.
- The next rivaroxaban dose is not to be administered earlier than 6 hours after the removal of the catheter.
- If traumatic puncture occurs, the administration of rivaroxaban is to be delayed for 24 hours.
Use in Patients with Renal Impairment
Nonvalvular Atrial Fibrillation
- Avoid the use of rivaroxaban in patients with CrCl <15 mL/min since drug exposure is increased.
- Periodically assess renal function as clinically indicated (i.e., more frequently in situations in which renal function may decline) and adjust therapy accordingly.
- Discontinue rivaroxaban in patients who develop acute renal failure while on rivaroxaban.
Treatment of Deep Vein Thrombosis (DVT), Pulmonary Embolism (PE), and Reduction in the Risk of Recurrence of DVT and of PE
- Avoid the use of rivaroxaban in patients with CrCl <30 mL/min due to an expected increase in rivaroxaban exposure and pharmacodynamic effects in this patient population.
Prophylaxis of Deep Vein Thrombosis Following Hip or Knee Replacement Surgery
- Avoid the use of rivaroxaban in patients with CrCl <30 mL/min due to an expected increase in rivaroxaban exposure and pharmacodynamic effects in this patient population.
- Observe closely and promptly evaluate any signs or symptoms of blood loss in patients with CrCl 30 to 50 mL/min.
- Patients who develop acute renal failure while on rivaroxaban should discontinue the treatment.
Use in Patients with Hepatic Impairment
- No clinical data are available for patients with severe hepatic impairment.
- Avoid use of rivaroxaban in patients with moderate (Child-Pugh B) and severe (Child-Pugh C) hepatic impairment or with any hepatic disease associated with coagulopathy since drug exposure and bleeding risk may be increased.
Use with P-gp and Strong CYP3A4 Inhibitors or Inducers
- Avoid concomitant use of rivaroxaban with combined P-gp and strong CYP3A4 inhibitors (e.g., ketoconazole, itraconazole, lopinavir/ritonavir, ritonavir, indinavir, and conivaptan).
- Avoid concomitant use of rivaroxaban with drugs that are combined P-gp and strong CYP3A4 inducers (e.g., carbamazepine, phenytoin, rifampin, St. John's wort).
Risk of Pregnancy-Related Hemorrhage
- In pregnant women, rivaroxaban should be used only if the potential benefit justifies the potential risk to the mother and fetus. rivaroxaban dosing in pregnancy has not been studied.
- The anticoagulant effect of rivaroxaban cannot be monitored with standard laboratory testing nor readily reversed.
- Promptly evaluate any signs or symptoms suggesting blood loss (e.g., a drop in hemoglobin and/or hematocrit, hypotension, or fetal distress).
Patients with Prosthetic Heart Valves
- The safety and efficacy of rivaroxaban have not been studied in patients with prosthetic heart valves. Therefore, use of rivaroxaban is not recommended in these patients.
Acute PE in Hemodynamically Unstable Patients or Patients Who Require Thrombolysis or Pulmonary Embolectomy
- Initiation of rivaroxaban is not recommended acutely as an alternative to unfractionated heparin in patients with pulmonary embolism who present with hemodynamic instability or who may receive thrombolysis or pulmonary embolectomy
Adverse Reactions
Clinical Trials Experience
During clinical development for the approved indications, 16326 patients were exposed to rivaroxaban. These included 7111 patients who received rivaroxaban 15 mg or 20 mg orally once daily for a mean of 19 months (5558 for 12 months and 2512 for 24 months) to reduce the risk of stroke and systemic embolism in nonvalvular atrial fibrillation (ROCKET AF); 4728 patients who received either rivaroxaban 15 mg orally twice daily for three weeks followed by 20 mg orally once daily (EINSTEIN DVT, EINSTEIN PE) or 20 mg orally once daily (EINSTEIN Extension) to treat DVT, PE, and to reduce the risk of recurrence of DVT and of PE; and 4487 patients who received rivaroxaban 10 mg orally once daily for prophylaxis of DVT following hip or knee replacement surgery (RECORD 1–3).
Hemorrhage
The most common adverse reactions with rivaroxaban were bleeding complications.
Nonvalvular Atrial Fibrillation
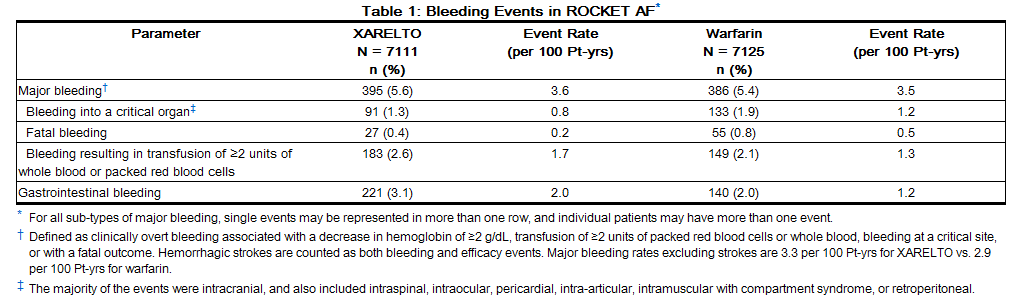
In the ROCKET AF trial, the most frequent adverse reactions associated with permanent drug discontinuation were bleeding events, with incidence rates of 4.3% for rivaroxaban vs. 3.1% for warfarin. The incidence of discontinuations for non-bleeding adverse events was similar in both treatment groups.
Table 1 shows the number of patients experiencing various types of bleeding events in the ROCKET AF trial.
Treatment of Deep Vein Thrombosis (DVT), Pulmonary Embolism (PE), and to Reduce the Risk of Recurrence of DVT and of PE
EINSTEIN DVT and EINSTEIN PE Studies
In the pooled analysis of the EINSTEIN DVT and EINSTEIN PE clinical studies, the most frequent adverse reactions leading to permanent drug discontinuation were bleeding events, with rivaroxaban vs. enoxaparin/Vitamin K antagonist (VKA) incidence rates of 1.7% vs. 1.5%, respectively. The mean duration of treatment was 208 days for rivaroxaban-treated patients and 204 days for enoxaparin/VKA-treated patients.
Table 2 shows the number of patients experiencing major bleeding events in the pooled analysis of the EINSTEIN DVT and EINSTEIN PE studies.

EINSTEIN Extension Study
In the EINSTEIN Extension clinical study, the most frequent adverse reactions associated with permanent drug discontinuation were bleeding events, with incidence rates of 1.8% for rivaroxaban vs. 0.2% for placebo treatment groups. The mean duration of treatment was 190 days for both rivaroxaban and placebo treatment groups.
Table 3 shows the number of patients experiencing bleeding events in the EINSTEIN Extension study.

Prophylaxis of Deep Vein Thrombosis Following Hip or Knee Replacement Surgery
In the RECORD clinical trials, the overall incidence rate of adverse reactions leading to permanent treatment discontinuation was 3.7% with rivaroxaban.
The rates of major bleeding events and any bleeding events observed in patients in the RECORD clinical trials are shown in Table 4.

Following rivaroxaban treatment, the majority of major bleeding complications (≥60%) occurred during the first week after surgery.
Other Adverse Reactions
Non-hemorrhagic adverse reactions reported in ≥1% of rivaroxaban-treated patients in the EINSTEIN Extension study are shown in Table 5.
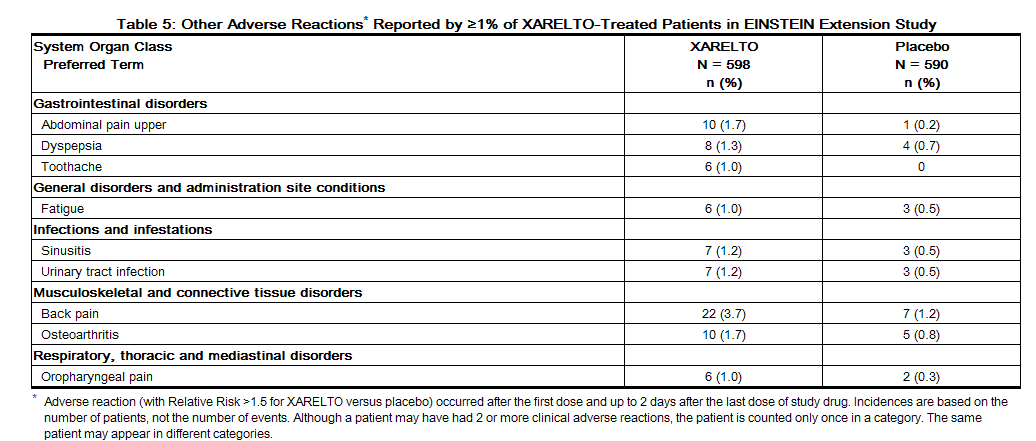
Non-hemorrhagic adverse reactions reported in ≥1% of rivaroxaban-treated patients in RECORD 1–3 studies are shown in Table 6.

Other clinical trial experience: In an investigational study of acute medically ill patients being treated with rivaroxaban 10 mg tablets, cases of pulmonary hemorrhage and pulmonary hemorrhage with bronchiectasis were observed.
Postmarketing Experience
The following adverse reactions have been identified during post-approval use of rivaroxaban. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Blood and lymphatic system disorders
Gastrointestinal disorders
Hepatobiliary disorders
- Jaundice
- Cholestasis
- Cytolytic hepatitis
Immune system disorders
Nervous system disorders
Skin and subcutaneous tissue disorders
Drug Interactions
Rivaroxaban is a substrate of CYP3A4/5, CYP2J2, and the P-gp and ATP-binding cassette G2 (ABCG2) transporters. Inhibitors and inducers of these CYP450 enzymes or transporters (e.g., P-gp) may result in changes in rivaroxaban exposure.
Drugs that Inhibit Cytochrome P450 3A4 Enzymes and Drug Transport Systems
- In drug interaction studies evaluating the concomitant use with drugs that are combined P-gp and CYP3A4 inhibitors (ketoconazole, ritonavir, clarithromycin, erythromycin and fluconazole), increases in rivaroxaban exposure and pharmacodynamic effects (i.e., factor Xa inhibition and PT prolongation) were observed.
- The increases in exposure ranged from 30% to 160%. Significant increases in rivaroxaban exposure may increase bleeding risk.
- When data suggest a change in exposure is unlikely to affect bleeding risk (e.g., clarithromycin, erythromycin), no precautions are necessary during coadministration with drugs that are combined P-gp and CYP3A4 inhibitors.
- Avoid concomitant administration of rivaroxaban with combined P-gp and strong CYP3A4 inhibitors.
Drugs that Induce Cytochrome P450 3A4 Enzymes and Drug Transport Systems
- Results from drug interaction studies and population PK analyses from clinical studies indicate coadministration of rivaroxaban with a combined P-gp and strong CYP3A4 inducer (e.g., rifampicin, phenytoin) decreased rivaroxaban exposure by up to 50%.
- Similar decreases in pharmacodynamic effects were also observed.
- These decreases in exposure to rivaroxaban may decrease efficacy.
- Avoid concomitant use of rivaroxaban with drugs that are combined P-gp and strong CYP3A4 inducers (e.g., carbamazepine, phenytoin, rifampin, St. John's wort).
Anticoagulants and NSAIDs/Aspirin
- Single doses of enoxaparin and rivaroxaban given concomitantly resulted in an additive effect on anti-factor Xa activity.
- Single doses of warfarin and rivaroxaban resulted in an additive effect on factor Xa (FXa) inhibition and PT.
- Concomitant aspirin use has been identified as an independent risk factor for major bleeding in efficacy trials.
- NSAIDs are known to increase bleeding, and bleeding risk may be increased when NSAIDs are used concomitantly with rivaroxaban.
- Coadministration of the platelet aggregation inhibitor clopidogrel and rivaroxaban resulted in an increase in bleeding time for some subjects.
- Avoid concurrent use of rivaroxaban with other anticoagulants due to increased bleeding risk unless benefit outweighs risk.
- Promptly evaluate any signs or symptoms of blood loss if patients are treated concomitantly with aspirin, other platelet aggregation inhibitors, or NSAIDs.
Drug-Disease Interactions with Drugs that Inhibit Cytochrome P450 3A4 Enzymes and Drug Transport Systems
- Results from a pharmacokinetic trial with erythromycin indicated that patients with renal impairment coadministered rivaroxaban with drugs classified as combined P-gp and moderate CYP3A4 inhibitors (e.g., diltiazem, verapamil, dronedarone, and erythromycin) have increased exposure compared with patients with normal renal function and no inhibitor use.
- Significant increases in rivaroxaban exposure may increase bleeding risk.
- While increases in rivaroxaban exposure can be expected under such conditions, results from an analysis in the ROCKET AF trial, which allowed concomitant use with combined P-gp and weak or moderate CYP3A4 inhibitors (e.g., amiodarone, diltiazem, verapamil, chloramphenicol, cimetidine, and erythromycin), did not show an increase in bleeding in patients with CrCl 30 to <50 mL/min [Hazard Ratio (95% CI): 1.05 (0.77, 1.42)].
- Rivaroxaban should not be used in patients with CrCl 15 to 80 mL/min who are receiving concomitant combined P-gp and moderate CYP3A4 inhibitors unless the potential benefit justifies the potential risk
Use in Specific Populations
Pregnancy
- There are no adequate or well-controlled studies of rivaroxaban in pregnant women, and dosing for pregnant women has not been established.
- Use rivaroxaban with caution in pregnant patients because of the potential for pregnancy related hemorrhage and/or emergent delivery with an anticoagulant that is not readily reversible.
- The anticoagulant effect of rivaroxaban cannot be reliably monitored with standard laboratory testing.
- Animal reproduction studies showed no increased risk of structural malformations, but increased post-implantation pregnancy loss occurred in rabbits.
- Rivaroxaban should be used during pregnancy only if the potential benefit justifies the potential risk to mother and fetus.
- Rivaroxaban crosses the placenta in animals. Animal reproduction studies have shown pronounced maternal hemorrhagic complications in rats and an increased incidence of post‑implantation pregnancy loss in rabbits.
- Rivaroxaban increased fetal toxicity (increased resorptions, decreased number of live fetuses, and decreased fetal body weight) when pregnant rabbits were given oral doses of ≥10 mg/kg rivaroxaban during the period of organogenesis.
- This dose corresponds to about 4 times the human exposure of unbound drug, based on AUC comparisons at the highest recommended human dose of 20 mg/day.
- Fetal body weights decreased when pregnant rats were given oral doses of 120 mg/kg. This dose corresponds to about 14 times the human exposure of unbound drug.
Pregnancy Category (AUS):
(Description)
Labor and Delivery
- Safety and effectiveness of rivaroxaban during labor and delivery have not been studied in clinical trials. However, in animal studies maternal bleeding and maternal and fetal death occurred at the rivaroxaban dose of 40 mg/kg (about 6 times maximum human exposure of the unbound drug at the human dose of 20 mg/day).
Nursing Mothers
- It is not known if rivaroxaban is excreted in human milk. Rivaroxaban and/or its metabolites were excreted into the milk of rats.
- Because many drugs are excreted in human milk and because of the potential for serious adverse reactions in nursing infants from rivaroxaban, a decision should be made whether to discontinue nursing or discontinue rovaroxaban, taking into account the importance of the drug to the mother.
Pediatric Use
- Safety and effectiveness in pediatric patients have not been established.
Geriatic Use
- Of the total number of patients in the RECORD 1–3 clinical studies evaluating rivaroxaban , about 54% were 65 years and over, while about 15% were >75 years. In ROCKET AF, approximately 77% were 65 years and over and about 38% were >75 years.
- In the EINSTEIN DVT, PE and Extension clinical studies approximately 37% were 65 years and over and about 16% were >75 years.
- In clinical trials the efficacy of rivaroxaban in the elderly (65 years or older) was similar to that seen in patients younger than 65 years.
- Both thrombotic and bleeding event rates were higher in these older patients, but the risk-benefit profile was favorable in all age groups
Gender
There is no FDA guidance on the use of Rivaroxaban with respect to specific gender populations.
Race
There is no FDA guidance on the use of Rivaroxaban with respect to specific racial populations.
Renal Impairment
Nonvalvular Atrial Fibrillation
In the ROCKET AF trial, patients with CrCl 30 to 50 mL/min were administered rivaroxaban 15 mg once daily resulting in serum concentrations of rivaroxaban and clinical outcomes similar to those in patients with better renal function administered rivaroxaban 20 mg once daily. Patients with CrCl 15 to 30 mL/min were not studied, but administration of rivaroxaban 15 mg once daily is also expected to result in serum concentrations of rivaroxaban similar to those in patients with normal renal function [see Dosage and Administration].
Treatment of DVT and/or PE, and Reduction in the Risk of Recurrence of DVT and of PE
In the EINSTEIN trials, patients with CrCl values <30 mL/min at screening were excluded from the studies. Avoid the use of rivaroxaban in patients with CrCl <30 mL/min.
Prophylaxis of DVT Following Hip or Knee Replacement Surgery
The combined analysis of the RECORD 1–3 clinical efficacy studies did not show an increase in bleeding risk for patients with CrCl 30 to 50 mL/min and reported a possible increase in total venous thromboemboli in this population. Observe closely and promptly evaluate any signs or symptoms of blood loss in patients with CrCl 30 to 50 mL/min. Avoid the use of rivaroxaban in patients with CrCl <30 mL/min.
Hepatic Impairment
(Description)
Females of Reproductive Potential and Males
- Females of reproductive potential requiring anticoagulation should discuss pregnancy planning with their physician.
Immunocompromised Patients
(Description)
Others
(Description)
Administration and Monitoring
Administration
(Oral/Intravenous/etc)
Monitoring
Condition 1
(Description regarding monitoring, from Warnings section)
Condition 2
(Description regarding monitoring, from Warnings section)
Condition 3
(Description regarding monitoring, from Warnings section)
IV Compatibility
Solution
Compatible
- Solution 1
- Solution 2
- Solution 3
Not Tested
- Solution 1
- Solution 2
- Solution 3
Variable
- Solution 1
- Solution 2
- Solution 3
Incompatible
- Solution 1
- Solution 2
- Solution 3
Y-Site
Compatible
- Solution 1
- Solution 2
- Solution 3
Not Tested
- Solution 1
- Solution 2
- Solution 3
Variable
- Solution 1
- Solution 2
- Solution 3
Incompatible
- Solution 1
- Solution 2
- Solution 3
Admixture
Compatible
- Solution 1
- Solution 2
- Solution 3
Not Tested
- Solution 1
- Solution 2
- Solution 3
Variable
- Solution 1
- Solution 2
- Solution 3
Incompatible
- Solution 1
- Solution 2
- Solution 3
Syringe
Compatible
- Solution 1
- Solution 2
- Solution 3
Not Tested
- Solution 1
- Solution 2
- Solution 3
Variable
- Solution 1
- Solution 2
- Solution 3
Incompatible
- Solution 1
- Solution 2
- Solution 3
TPN/TNA
Compatible
- Solution 1
- Solution 2
- Solution 3
Not Tested
- Solution 1
- Solution 2
- Solution 3
Variable
- Solution 1
- Solution 2
- Solution 3
Incompatible
- Solution 1
- Solution 2
- Solution 3
Overdosage
- Overdose of rivaroxaban may lead to hemorrhage.
- Discontinue rivaroxaban and initiate appropriate therapy if bleeding complications associated with overdosage occur.
- A specific antidote for rivaroxaban is not available.
- Rivaroxaban systemic exposure is not further increased at single doses >50 mg due to limited absorption.
- The use of activated charcoal to reduce absorption in case of rivaroxaban overdose may be considered.
- Due to the high plasma protein binding, rivaroxaban is not expected to be dialyzable.
- Partial reversal of laboratory anticoagulation parameters may be achieved with use of plasma products.
Pharmacology
Mechanism of Action
(Description)
Structure
(Description with picture)
Pharmacodynamics
(Description)
Pharmacokinetics
(Description)
Nonclinical Toxicology
(Description)
Clinical Studies
Condition 1
(Description)
Condition 2
(Description)
Condition 3
(Description)
How Supplied
(Description)
Storage
There is limited information regarding Rivaroxaban Storage in the drug label.
Images
Drug Images
{{#ask: Page Name::Rivaroxaban |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
{{#ask: Label Page::Rivaroxaban |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
(Patient Counseling Information)
Precautions with Alcohol
Alcohol-Rivaroxaban interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
There is limited information regarding Rivaroxaban Brand Names in the drug label.
Look-Alike Drug Names
- (Paired Confused Name 1a) — (Paired Confused Name 1b)
- (Paired Confused Name 2a) — (Paired Confused Name 2b)
- (Paired Confused Name 3a) — (Paired Confused Name 3b)
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.