Phosphoric acids and phosphates
Editor-In-Chief: Henry A. Hoff
Of the many phosphorus oxoacids, this article compares various kinds of phosphoric acids and phosphates.
Orthophosphoric acid
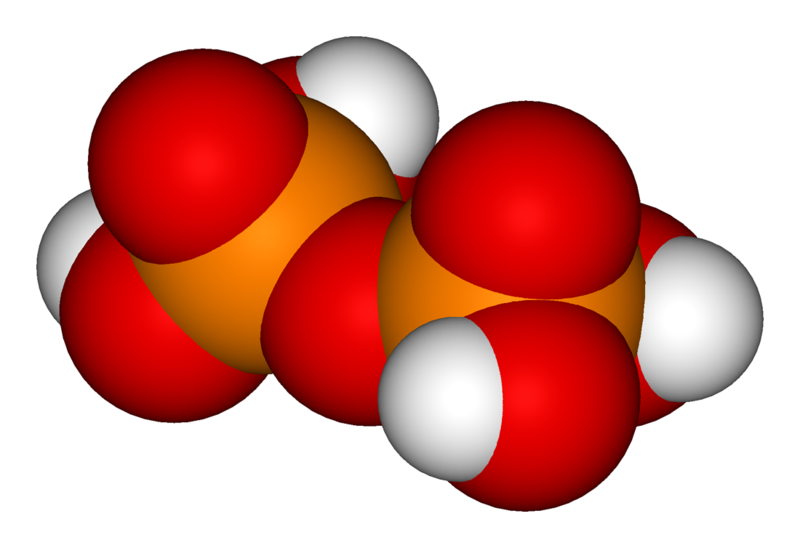
The simplest compound of a series of phosphoric acids is sometimes called by its common name, orthophosphoric acid, but more often called by its IUPAC name, simply phosphoric acid, by both non-technical people and even many chemists. It has also been called monophosphoric acid.[1] The chemical formula of orthophosphoric acid is H3PO4 and its chemical structure is labeled in the illustration. There is a separate article on this most important compound in the series at: Phosphoric acid. However, two or more orthophosphoric acid molecules can be joined by condensation into larger molecules by elimination of water. This way, a series of polyphosphoric acids can be obtained.
-
orthophosphoric acid
H3PO4 -
pyrophosphoric acid
H4P2O7 -
tripolyphosphoric acid
H5P3O10 -
tetrapolyphosphoric acid
H6P4O13 -
trimetaphosphoric acid
H3P3O9 -
phosphoric anhydride
P4O10
Orthophosphate
Orthophosphoric acid has three hydrogen atoms bonded to oxygen atoms in its structure. All three hydrogens are acidic to varying degrees and can be lost from the molecule as H+ ions (alternatively referred to as protons). When all three H+ ions are lost from orthophosphoric acid, an orthophosphate ion (PO43-) is formed. Orthophosphate is the simplest in a series of phosphates, and is usually just called phosphate by both non-technical people and many chemists alike; see a separate article on phosphate for details.
Because orthophosphoric acid can undergo as many as three dissociations or ionizations (losses of H+ ions), it has three acid dissociation constants called Ka1, Ka2, and Ka3. Another way to provide acid dissociation constant data is to list pKa1, pKa2, and pKa3 instead. Orthophosphate is in a sense the triple conjugate base of phosphoric acid and has three related basicity constants, Kb1, Kb2, and Kb3, which likewise have corresponding pKb1, pKb2, and pKb3 values.
Polyphosphoric acids
When two orthophosphoric acid molecules are condensed into one molecule, pyrophosphoric acid (H4P2O7) is obtained as follows:
- 2 H3PO4 → H4P2O7 + H2O
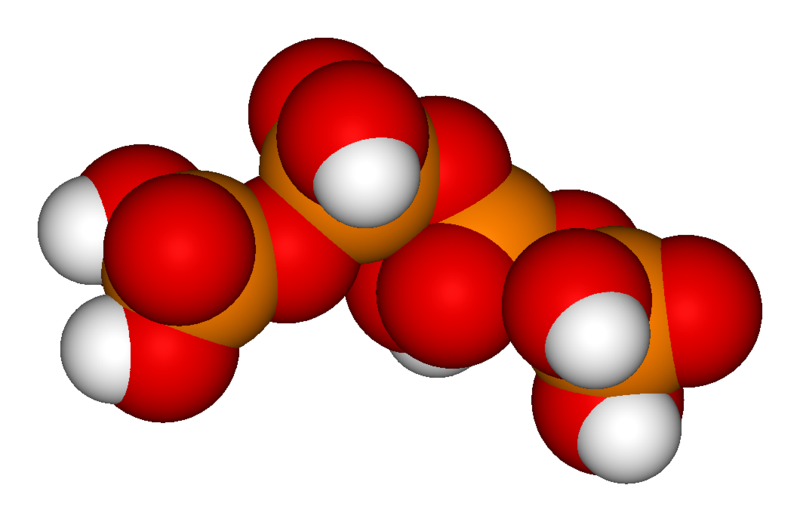
The chemical structure of pyrophosphoric acid is also shown in the illustration. There is also a separate article on Pyrophosphoric acid. Three orthophosphoric acid molecules can condense in a row to obtain tripolyphosphoric acid (H5P3O10), which is also shown in the illustration and has a separate article: Triphosphoric acid. This condensation process can continue with additional orthophosphoric acid units to obtain tetrapolyphosphoric acid (H6P4O13, pictured) and so on. Note that each extra phosphoric unit adds 1 extra H (hydrogen) atom, 1 extra P (phosphorus) atom, and 3 extra O (oxygen) atoms. The "backbone" chain of these types of molecules consists of alternating P and O atoms covalently bonded together. The term oligophosphoric acid refers to this series of phosphoric acids when a small number of phosphoric acids are joined, per the general formula Hn+2PnO3n+1, if n = 3, 4, 5, etc., oligo-.[2] Alternately, the above series follows the naming pattern of di- (pyrophosphoric), tri-, tetra-, penta-, hexaphosphoric acid, etc. When n is very large, the polymer is polyphosphoric acid.[2] Polyphosphoric acid molecules can have dozens of such phosphoric units bonded in a row. A general formula for such poly-acid compounds is HO(PO2OH)xH, where x = number of phosphoric units in the molecule. The four oxygen atoms bonded to each phosphorus atom are in a tetrahedral configuration with the phosphorus in the center of the tetrahedron and the oxygens in each of the four corners.
Polyphosphates
In a pyrophosphoric acid molecule, there are four hydrogens bonded to oxygens, and one, two, three, or all four can be lost as H+ ions. When all four are lost from pyrophosphoric acid, a pyrophosphate ion is formed. Because pyrophosphoric acids can undergo four dissociations, there are four Ka values for it, as well as four corresponding pKa values. Similarly, pyrophosphate is a base with four Kb and, of course, four pKb values for regaining the H+ ions in reverse order.
The situation with higher order polyphosphoric acids and polyphosphates continues in a similar way. Tripolyphosphoric acid can lose up to five H+ ions to form a tripolyphosphate ion, tetrapolyphosphoric acid can lose up to six H+ ions to form tetrapolyphosphate, etc. As more dissociations per molecule are possible, the intervals between individual pKa and pKb values now start becoming smaller on the pH scale.
As the polyphosphoric molecules grow increasingly larger and more complex, practically any number of the somewhat acidic -OH groups in them can dissociate to become negatively charged oxygens, forming numerous combinations of multiple-charged polyphosphoric/polyphosphate anions. Generally in an aqueous solution, the degree or percentage of dissociation depends on the pH of the solution.
Ortho-, pyro-, and tripolyphosphate compounds have been commonly used in detergents (i. e. cleaners) formulations. For example, see Sodium tripolyphosphate. Sometimes pyrophosphate, tripolyphosphate, tetrapolyphosphate, etc. are called diphosphate, triphosphate, tetraphosphate, etc., especially when they are part of phosphate esters in biochemistry.
Cyclo- or metaphosphoric acids and metaphosphates
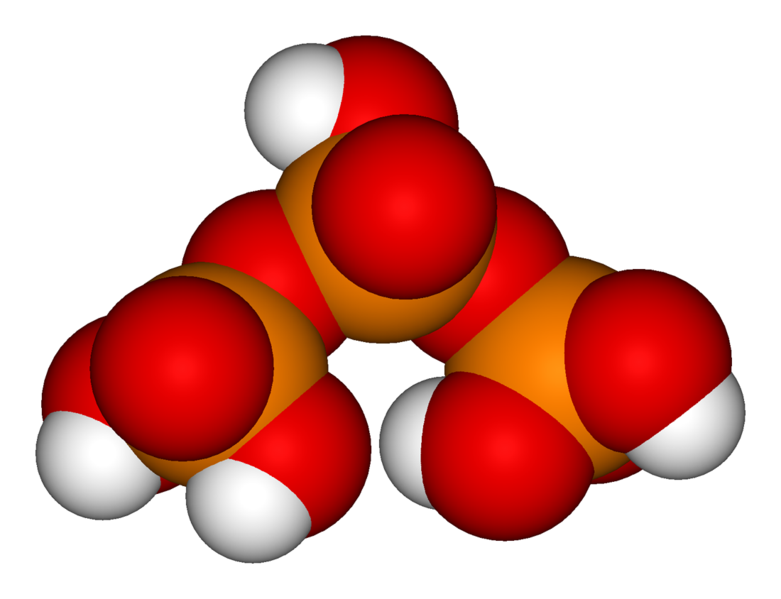
Phosphoric acid units can be bonded together in rings (cyclic structures) formed of metaphosphoric acid molecules. See metaphosphoric acid. The simplest such compound is trimetaphosphoric acid or cyclo-triphosphoric acid having the formula H3P3O9. Its structure is shown in the illustration. Since the ends are condensed, its formula has one less H2O (water) than tripolyphosphoric acid. A general formula for such cyclic compounds is (HPO3)x where x = number of phosphoric units in the molecule.
When these metaphosphoric acids lose their hydrogens as H+, cyclic anions called metaphosphates are formed. An example of a compound with such an anion is sodium hexametaphosphate (Na6P6O18) used as a sequestrant and a food additive.
Branched polyphosphates
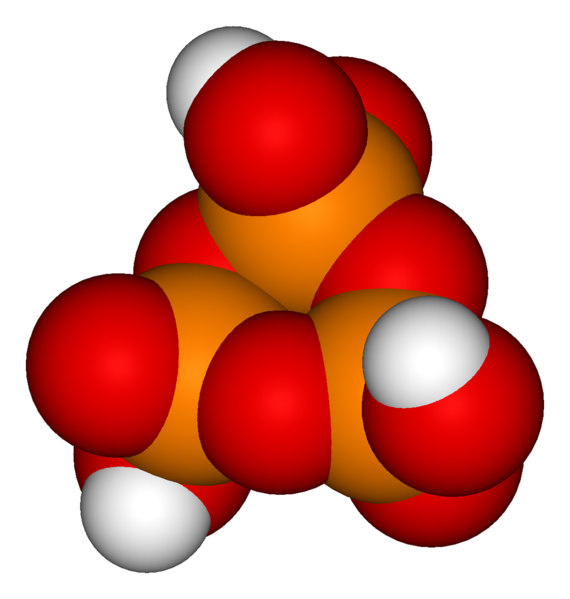
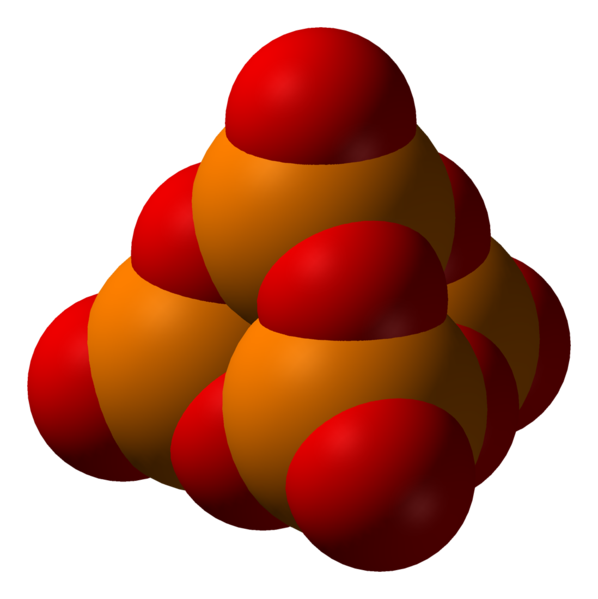
The third -OH group on an orthophosphoric acid unit can also be used for condensation with other phosphoric groups to form branches in the polyphosphoric/polyphosphate chains. The ultimate example of cyclic and branching condensation would be the cyclic four-phosphate unit double-branched to form the phosphoric anhydride P4O10; see illustration. Polyphosphoric acids that are also branched can be referred to as ultraphosphoric acid or ultrapolyphosphoric acid[3].
Hydrolysis of polyphosphoric/polyphosphates
These phosphoric acids series are generally water-soluble considering the polarity of the molecules. Ammonium and alkali phosphates are also quite soluble in water. The alkaline earth salts start becoming less soluble and phosphate salts of various other metals are even less soluble. In aqueous solutions (solutions of water), water gradually (over the course of hours) hydrolyzes polyphosphates into smaller phosphates and finally into ortho-phosphate, given enough water. Higher temperature or acidic conditions can speed up the hydrolysis reactions considerably.
Conversely, polyphosphoric acids or polyphosphates are often formed by dehydrating a phosphoric acid solution; in other words, removing water from it often by heating and evaporating the water off.
Phosphate and phosphite esters

Here any R can be H
or some other organic radical
The -OH groups in phosphoric acids can also condense with the hydroxyl groups of alcohols to form phosphate esters. Since orthophosphoric acid has three -OH groups, it can esterify with one, two, or three alcohol molecules to form a mono-, di-, or triester. See the general structure image of an ortho- (or mono-) phosphate ester below on the left, where any of the R groups can be a hydrogen or an organic radical. Pyro- (or di-) phosphate esters and tripoly- (or tri-) phosphate esters, etc. are also possible. Any -OH groups on the phosphates in these ester molecules may lose H+ ions to form anions, again depending on the pH in a solution. In the biochemistry of living organisms, there are many kinds of (mono)phosphate, diphosphate, and triphosphate compounds (essentially esters), many of which play a significant role in metabolism such as adenosine diphosphate (ADP) and triphosphate (ATP).

Here any R can be H
or some other organic radical
Similarly, phosphorous acid can bond with alcohol molecules to form a phosphite ester. See the general structure image below on the right. The two dots on the P represent the lone electron pair of the phosphorus atom.
References
- ↑ Robertson DS (2004). "A possible natural antibacterial compound in the human metabolism". Med Hypotheses. 63 (3): 554–5. PMID 15288387.
- ↑ 2.0 2.1 Wiberg E, Wiberg N, Holleman AF (2001). Inorganic chemistry. Academic Press. p. 727-8. ISBN 0123526515, 9780123526519 Check
|isbn=value: invalid character (help). More than one of|pages=and|page=specified (help) - ↑ Shiba T, Shiba T, Yamaoka M, Uematsu T, Takahashi Y, Tanaka H, Kogo T, Shindo M. "Antiinflammtory agent and antiinflammatory medical material". Unknown parameter
|USPTO Application #=ignored (help)