Phosphoric acids and phosphates: Difference between revisions
No edit summary |
m (→References) |
||
| (One intermediate revision by the same user not shown) | |||
| Line 3: | Line 3: | ||
'''Editor-In-Chief:''' Henry A. Hoff | '''Editor-In-Chief:''' Henry A. Hoff | ||
Of the many [[phosphorus oxoacids]], the '''phosphoric acids''' constitute the largest and most diverse group. The simplest phosphoric acid series begins with monophosphoric (orthophosphoric) acid, diphosphoric (pyrophosphoric) acid | Of the many [[phosphorus oxoacids]], the '''phosphoric acids''' constitute the largest and most diverse group. The simplest phosphoric acid series begins with monophosphoric (orthophosphoric) acid, continues with many oligophosphoric acids such as diphosphoric (pyrophosphoric) acid and concludes in the polyphorphoric acids. But phosphoric acid units can bind together into rings or cyclic structures, chains (catenas), or branched structures, with various combinations possible. Each of these can form phosphates (salts or esters). | ||
Phosphorus can be further oxidized while in the +5 state to paraphosphoric acid (H<sub>5</sub>PO<sub>5</sub>). Occasionally oxygen can be replaced by sulfur to form thiophosphoric acids. When the acid contains one more oxygen (+7 state) than phosphoric acid it is called '''perphosphoric acid'''. | Phosphorus can be further oxidized while in the +5 state to paraphosphoric acid (H<sub>5</sub>PO<sub>5</sub>). Occasionally oxygen can be replaced by sulfur to form thiophosphoric acids. When the acid contains one more oxygen (+7 state for phosphorus) than phosphoric acid, it is called '''perphosphoric acid'''. | ||
When commercially produced for fertilizers, a blend of phosphoric acids is used and termed '''superphosphoric acid'''. Further concentrating of phosphoric acid results in hyperphosphoric acid. | When commercially produced for fertilizers, a blend of phosphoric acids is used and termed '''superphosphoric acid'''. Further concentrating of phosphoric acid results in hyperphosphoric acid. | ||
| Line 30: | Line 30: | ||
Because orthophosphoric acid can undergo as many as three dissociations or ionizations (losses of H<sup>+</sup> ions), H<sub>2</sub>PO<sub>4</sub><sup>1-</sup>, dihydrogen phosphate ion, HPO<sub>4</sub><sup>2-</sup>, hydrogen phosphate ion, and PO<sub>4</sub><sup>3-</sup>, phosphate ion, it has three [[acid dissociation constant]]s called K<sub>a1</sub>, K<sub>a2</sub>, and K<sub>a3</sub>. Another way to provide acid dissociation constant data is to list pK<sub>a1</sub>, pK<sub>a2</sub>, and pK<sub>a3</sub> instead. Orthophosphate is in a sense the triple [[conjugate base]] of phosphoric acid and has three related [[Acid dissociation constant#Bases|basicity constant]]s, K<sub>b1</sub>, K<sub>b2</sub>, and K<sub>b3</sub>, which likewise have corresponding pK<sub>b1</sub>, pK<sub>b2</sub>, and pK<sub>b3</sub> values. | Because orthophosphoric acid can undergo as many as three dissociations or ionizations (losses of H<sup>+</sup> ions), H<sub>2</sub>PO<sub>4</sub><sup>1-</sup>, dihydrogen phosphate ion, HPO<sub>4</sub><sup>2-</sup>, hydrogen phosphate ion, and PO<sub>4</sub><sup>3-</sup>, phosphate ion, it has three [[acid dissociation constant]]s called K<sub>a1</sub>, K<sub>a2</sub>, and K<sub>a3</sub>. Another way to provide acid dissociation constant data is to list pK<sub>a1</sub>, pK<sub>a2</sub>, and pK<sub>a3</sub> instead. Orthophosphate is in a sense the triple [[conjugate base]] of phosphoric acid and has three related [[Acid dissociation constant#Bases|basicity constant]]s, K<sub>b1</sub>, K<sub>b2</sub>, and K<sub>b3</sub>, which likewise have corresponding pK<sub>b1</sub>, pK<sub>b2</sub>, and pK<sub>b3</sub> values. | ||
== | ==Oligophosphoric acids== | ||
When two orthophosphoric acid molecules are condensed into one molecule, '''pyrophosphoric acid''' (H<sub>4</sub>P<sub>2</sub>O<sub>7</sub>) is obtained as follows: | When two orthophosphoric acid molecules are condensed into one molecule, '''pyrophosphoric acid''' (H<sub>4</sub>P<sub>2</sub>O<sub>7</sub>) is obtained as follows: | ||
| Line 36: | Line 36: | ||
::::::2 H<sub>3</sub>PO<sub>4</sub> → H<sub>4</sub>P<sub>2</sub>O<sub>7</sub> + H<sub>2</sub>O | ::::::2 H<sub>3</sub>PO<sub>4</sub> → H<sub>4</sub>P<sub>2</sub>O<sub>7</sub> + H<sub>2</sub>O | ||
The chemical structure of pyrophosphoric acid is also shown in the illustration. There is also a separate article on [[Pyrophosphoric acid]]. Three orthophosphoric acid molecules can condense in a row to obtain '''tripolyphosphoric acid''' (H<sub>5</sub>P<sub>3</sub>O<sub>10</sub>), which is also shown in the illustration and has a separate article: [[Triphosphoric acid]]. This condensation process can continue with additional orthophosphoric acid units to obtain '''tetrapolyphosphoric acid''' (H<sub>6</sub>P<sub>4</sub>O<sub>13</sub>, pictured) and so on. Note that each extra phosphoric unit adds 1 extra H ([[hydrogen]]) atom, 1 extra P ([[phosphorus]]) atom, and 3 extra O ([[oxygen]]) atoms. The "backbone" chain of these types of molecules consists of alternating P and O atoms [[covalent bond|covalently bonded]] together. The term '''oligophosphoric acid''' refers to this series of phosphoric acids when a small number of phosphoric acids are joined, per the general formula H<sub>n+2</sub>P<sub>n</sub>O<sub>3n+1</sub>, if n = 3, 4, 5, etc., oligo-.<ref name=Wiberg>{{ cite book |author=Wiberg E, Wiberg N, Holleman AF |year=2001 |page=727-8 |pages=1884 |title=Inorganic chemistry |publisher=Academic Press |ISBN=0123526515, 9780123526519 }}</ref> Alternately, the above series follows the naming pattern of di- (pyrophosphoric), tri-, tetra-, penta-, hexaphosphoric acid, etc | The chemical structure of pyrophosphoric acid is also shown in the illustration. There is also a separate article on [[Pyrophosphoric acid]]. Three orthophosphoric acid molecules can condense in a row to obtain '''tripolyphosphoric acid''' (H<sub>5</sub>P<sub>3</sub>O<sub>10</sub>), which is also shown in the illustration and has a separate article: [[Triphosphoric acid]]. This condensation process can continue with additional orthophosphoric acid units to obtain '''tetrapolyphosphoric acid''' (H<sub>6</sub>P<sub>4</sub>O<sub>13</sub>, pictured) and so on. Note that each extra phosphoric unit adds 1 extra H ([[hydrogen]]) atom, 1 extra P ([[phosphorus]]) atom, and 3 extra O ([[oxygen]]) atoms. The "backbone" chain of these types of molecules consists of alternating P and O atoms [[covalent bond|covalently bonded]] together. The term '''oligophosphoric acid''' refers to this series of phosphoric acids when a small number of phosphoric acids are joined, per the general formula H<sub>n+2</sub>P<sub>n</sub>O<sub>3n+1</sub>, if n = 3, 4, 5, etc., oligo-.<ref name=Wiberg>{{ cite book |author=Wiberg E, Wiberg N, Holleman AF |year=2001 |page=727-8 |pages=1884 |title=Inorganic chemistry |publisher=Academic Press |ISBN=0123526515, 9780123526519 }}</ref> Alternately, the above series follows the naming pattern of di- (pyrophosphoric), tri-, tetra-, penta-, hexaphosphoric acid, etc. | ||
== | ==Oligophosphates== | ||
In a pyrophosphoric acid molecule, there are four hydrogens bonded to oxygens, and one, two, three, or all four can be lost as H<sup>+</sup> ions. When all four are lost from pyrophosphoric acid, a '''pyrophosphate''' ion is formed. Because pyrophosphoric acids can undergo four dissociations, there are four K<sub>a</sub> values for it, as well as four corresponding pK<sub>a</sub> values. Similarly, pyrophosphate is a base with four K<sub>b</sub> and, of course, four pK<sub>b</sub> values for regaining the H<sup>+</sup> ions in reverse order. | In a pyrophosphoric acid molecule, there are four hydrogens bonded to oxygens, and one, two, three, or all four can be lost as H<sup>+</sup> ions. When all four are lost from pyrophosphoric acid, a '''pyrophosphate''' ion is formed. Because pyrophosphoric acids can undergo four dissociations, there are four K<sub>a</sub> values for it, as well as four corresponding pK<sub>a</sub> values. Similarly, pyrophosphate is a base with four K<sub>b</sub> and, of course, four pK<sub>b</sub> values for regaining the H<sup>+</sup> ions in reverse order. | ||
The situation with higher order | The situation with higher order oligophosphoric acids and oligophosphates continues in a similar way. Tripolyphosphoric acid can lose up to five H<sup>+</sup> ions to form a '''tripolyphosphate''' ion, tetrapolyphosphoric acid can lose up to six H<sup>+</sup> ions to form '''tetrapolyphosphate''', etc. As more dissociations per molecule are possible, the intervals between individual pK<sub>a</sub> and pK<sub>b</sub> values now start becoming smaller on the [[pH]] scale. | ||
Ortho-, pyro-, and tripolyphosphate compounds have been commonly used in [[detergent]]s (i. e. cleaners) formulations. For example, see [[Sodium tripolyphosphate]]. Sometimes pyrophosphate, tripolyphosphate, tetrapolyphosphate, etc. are called '''diphosphate''', '''triphosphate''', '''tetraphosphate''', etc., especially when they are part of [[phosphate ester]]s in [[biochemistry]]. | |||
==Polyphosphoric acids== | |||
When n is very large, the polymer is polyphosphoric acid.<ref name=Wiberg/> Polyphosphoric acid molecules can have dozens of such phosphoric units bonded in a row. A general formula for such poly-acid compounds is HO(PO<sub>2</sub>OH)<sub>x</sub>H, where x = number of phosphoric units in the molecule. The four oxygen atoms bonded to each phosphorus atom are in a tetrahedral configuration with the phosphorus in the center of the [[tetrahedron]] and the oxygens in each of the four corners. | |||
==Polyphosphates== | |||
As the polyphosphoric molecules grow increasingly larger and more complex, practically any number of the somewhat acidic -OH groups in them can dissociate to become negatively charged oxygens, forming numerous combinations of multiple-charged polyphosphoric/polyphosphate anions. Generally in an [[aqueous]] [[solution]], the degree or percentage of dissociation depends on the [[pH]] of the solution. | As the polyphosphoric molecules grow increasingly larger and more complex, practically any number of the somewhat acidic -OH groups in them can dissociate to become negatively charged oxygens, forming numerous combinations of multiple-charged polyphosphoric/polyphosphate anions. Generally in an [[aqueous]] [[solution]], the degree or percentage of dissociation depends on the [[pH]] of the solution. | ||
==Cyclo- or metaphosphoric acids and metaphosphates== | ==Cyclo- or metaphosphoric acids and metaphosphates== | ||
| Line 67: | Line 73: | ||
==Perphosphoric acid== | ==Perphosphoric acid== | ||
[[Image:Permonophosphoric Acid1.jpg|thumb|120px|left|Permonophosphoric Acid]] | [[Image:Permonophosphoric Acid1.jpg|thumb|120px|left|Permonophosphoric Acid]] | ||
The use of '''per''' before the '''phosphoric''' indicates that the acid contains one more oxygen than phosphoric acid. For example, permonophosphoric acid has the formula H<sub>3</sub>PO<sub>5</sub> instead of H<sub>3</sub>PO<sub>4</sub> for orthophosphoric acid (monophosphoric acid). | The use of '''per''' before the '''phosphoric''' indicates that the acid contains one more oxygen than phosphoric acid. For example, permonophosphoric acid has the formula H<sub>3</sub>PO<sub>5</sub> instead of H<sub>3</sub>PO<sub>4</sub> for orthophosphoric acid (monophosphoric acid). | ||
| Line 79: | Line 86: | ||
Monoperphosphoric acid and permonophosphoric acid are the same acid. Should the phosphoric acid be either an oligophosphoric acid or polyphosphoric acid as discussed below, the location of '''per''' may indicate different acids. For instance, '''per'''diphosphoric acid would be H<sub>4</sub>P<sub>2</sub>O<sub>8</sub>, one more oxygen added to pyrophosphoric acid. But di'''per'''phosphoric acid could refer to two permonophosphoric acid molecules condensed with the loss of H<sub>2</sub>O to yield H<sub>4</sub>P<sub>2</sub>O<sub>9</sub>. | Monoperphosphoric acid and permonophosphoric acid are the same acid. Should the phosphoric acid be either an oligophosphoric acid or polyphosphoric acid as discussed below, the location of '''per''' may indicate different acids. For instance, '''per'''diphosphoric acid would be H<sub>4</sub>P<sub>2</sub>O<sub>8</sub>, one more oxygen added to pyrophosphoric acid. But di'''per'''phosphoric acid could refer to two permonophosphoric acid molecules condensed with the loss of H<sub>2</sub>O to yield H<sub>4</sub>P<sub>2</sub>O<sub>9</sub>. | ||
==Perphosphates== | |||
Perphosphates have been produced from perphosphoric acid.<ref name=Jones>{{ cite journal |author=Jones NC |title=The anode reactions of fluorine |journal=J Phys Chem. |year=1929 |volume=33 |month=Jan |issue=6 |pages=801–24 |doi=10.1021/j150300a001 }}</ref> | Perphosphates have been produced from perphosphoric acid.<ref name=Jones>{{ cite journal |author=Jones NC |title=The anode reactions of fluorine |journal=J Phys Chem. |year=1929 |volume=33 |month=Jan |issue=6 |pages=801–24 |doi=10.1021/j150300a001 }}</ref> | ||
| Line 87: | Line 96: | ||
Superphosphoric acid is sometimes called '''multiphosphoric acid''' or '''polyphosphoric acid'''. | Superphosphoric acid is sometimes called '''multiphosphoric acid''' or '''polyphosphoric acid'''. | ||
==Superphosphates== | |||
[[Superphosphate]]s (salts or esters) can result from the use of superphosphoric acid. Superphosphate has been produced by acidulating phosphate rock with superphosphoric acid, where the superphosphoric acid contained about 49% of its phosphate as orthophosphate, 42% as pyrophosphate, and the remainder as polyphosphates.<ref name=Phillips>{{ cite journal |author=Phillips AB, Young RD, Heil FG, Norton MM |title=Fertilizer technology, high-analysis superphosphate by the reaction of phosphate rock with superphosphoric acid |journal=J Agric Food Chem. |month=Jul |year=1960 |volume=8 |issue=4 |pages=310-5 |doi=10.1021/jf60110a016 }}</ref> | [[Superphosphate]]s (salts or esters) can result from the use of superphosphoric acid. Superphosphate has been produced by acidulating phosphate rock with superphosphoric acid, where the superphosphoric acid contained about 49% of its phosphate as orthophosphate, 42% as pyrophosphate, and the remainder as polyphosphates.<ref name=Phillips>{{ cite journal |author=Phillips AB, Young RD, Heil FG, Norton MM |title=Fertilizer technology, high-analysis superphosphate by the reaction of phosphate rock with superphosphoric acid |journal=J Agric Food Chem. |month=Jul |year=1960 |volume=8 |issue=4 |pages=310-5 |doi=10.1021/jf60110a016 }}</ref> | ||
| Line 103: | Line 114: | ||
Hyperphosphoric acid can be added to blood to release CO<sub>2</sub>.<ref name=Sidossis>{{ cite journal |author=Sidossis LS, Mittendorfer B, Chinkes D, Walser E, Wolfe RR |title=Effect of hyperglycemia-hyperinsulinemia on whole body and regional fatty acid metabolism |journal=Am J Physiol. |volume=276 |pages=E427-34 |year=1999 |month=Mar |issue=(3 Pt 1) |pmid=10070006 }}</ref> | Hyperphosphoric acid can be added to blood to release CO<sub>2</sub>.<ref name=Sidossis>{{ cite journal |author=Sidossis LS, Mittendorfer B, Chinkes D, Walser E, Wolfe RR |title=Effect of hyperglycemia-hyperinsulinemia on whole body and regional fatty acid metabolism |journal=Am J Physiol. |volume=276 |pages=E427-34 |year=1999 |month=Mar |issue=(3 Pt 1) |pmid=10070006 }}</ref> | ||
==Hyperphosphates== | |||
Hyperphosphates (salts or esters) or oleophosphates can result from the use of hyperphosphoric acid. Hyperphosphate, when used to induce [[hyperphosphatemia]], may directly promote calcium deposition and [[osteocalcin]] expression.<ref name=Lu>{{ cite journal |author=Lu W, Wang X, Zhao X |title=Study of vascular smooth muscle cell calcification induced by hyperphosphate and intervented by phosphonoformic acid |year=2007 |journal=J Nanjing Med Univ. |volume=21 |issue=6 |month=Nov |pages=377-81 |doi=10.1016/S1007-4376(07)60082-3 }}</ref> | Hyperphosphates (salts or esters) or oleophosphates can result from the use of hyperphosphoric acid. Hyperphosphate, when used to induce [[hyperphosphatemia]], may directly promote calcium deposition and [[osteocalcin]] expression.<ref name=Lu>{{ cite journal |author=Lu W, Wang X, Zhao X |title=Study of vascular smooth muscle cell calcification induced by hyperphosphate and intervented by phosphonoformic acid |year=2007 |journal=J Nanjing Med Univ. |volume=21 |issue=6 |month=Nov |pages=377-81 |doi=10.1016/S1007-4376(07)60082-3 }}</ref> | ||
| Line 126: | Line 139: | ||
*[http://www1.dionex.com/en-us/webdocs/application/industry/chempetro/AN71_V18.pdf Determination of Polyphosphates Using Ion Chromatography with Suppressed Conductivity Detection, Application Note 71 by Dionex] | *[http://www1.dionex.com/en-us/webdocs/application/industry/chempetro/AN71_V18.pdf Determination of Polyphosphates Using Ion Chromatography with Suppressed Conductivity Detection, Application Note 71 by Dionex] | ||
{{Phosphate biochemistry}} | |||
[[Category:Phosphates]] | [[Category:Phosphates]] | ||
Latest revision as of 02:23, 17 February 2020
Editor-In-Chief: Henry A. Hoff
Of the many phosphorus oxoacids, the phosphoric acids constitute the largest and most diverse group. The simplest phosphoric acid series begins with monophosphoric (orthophosphoric) acid, continues with many oligophosphoric acids such as diphosphoric (pyrophosphoric) acid and concludes in the polyphorphoric acids. But phosphoric acid units can bind together into rings or cyclic structures, chains (catenas), or branched structures, with various combinations possible. Each of these can form phosphates (salts or esters).
Phosphorus can be further oxidized while in the +5 state to paraphosphoric acid (H5PO5). Occasionally oxygen can be replaced by sulfur to form thiophosphoric acids. When the acid contains one more oxygen (+7 state for phosphorus) than phosphoric acid, it is called perphosphoric acid.
When commercially produced for fertilizers, a blend of phosphoric acids is used and termed superphosphoric acid. Further concentrating of phosphoric acid results in hyperphosphoric acid.
Orthophosphoric acid
The simplest compound of a series of phosphoric acids is sometimes called by its common name, orthophosphoric acid, but more often called by its IUPAC name, simply phosphoric acid, by both non-technical people and even many chemists. It has also been called monophosphoric acid.[1] The chemical formula of orthophosphoric acid is H3PO4 and its chemical structure is labeled in the illustration. There is a separate article on this most important compound in the series at: Phosphoric acid. However, two or more orthophosphoric acid molecules can be joined by condensation into larger molecules by elimination of water. This way, a series of polyphosphoric acids can be obtained.
-
orthophosphoric acid
H3PO4 -
pyrophosphoric acid
H4P2O7 -
tripolyphosphoric acid
H5P3O10 -
tetrapolyphosphoric acid
H6P4O13 -
trimetaphosphoric acid
H3P3O9 -
phosphoric anhydride
P4O10
Orthophosphate
Orthophosphoric acid has three hydrogen atoms bonded to oxygen atoms in its structure. All three hydrogens are acidic to varying degrees and can be lost from the molecule as H+ ions (alternatively referred to as protons). When all three H+ ions are lost from orthophosphoric acid, an orthophosphate ion (PO43-) is formed. Orthophosphate is the simplest in a series of phosphates, and is usually just called phosphate by both non-technical people and many chemists alike; see a separate article on phosphate for details.
Because orthophosphoric acid can undergo as many as three dissociations or ionizations (losses of H+ ions), H2PO41-, dihydrogen phosphate ion, HPO42-, hydrogen phosphate ion, and PO43-, phosphate ion, it has three acid dissociation constants called Ka1, Ka2, and Ka3. Another way to provide acid dissociation constant data is to list pKa1, pKa2, and pKa3 instead. Orthophosphate is in a sense the triple conjugate base of phosphoric acid and has three related basicity constants, Kb1, Kb2, and Kb3, which likewise have corresponding pKb1, pKb2, and pKb3 values.
Oligophosphoric acids
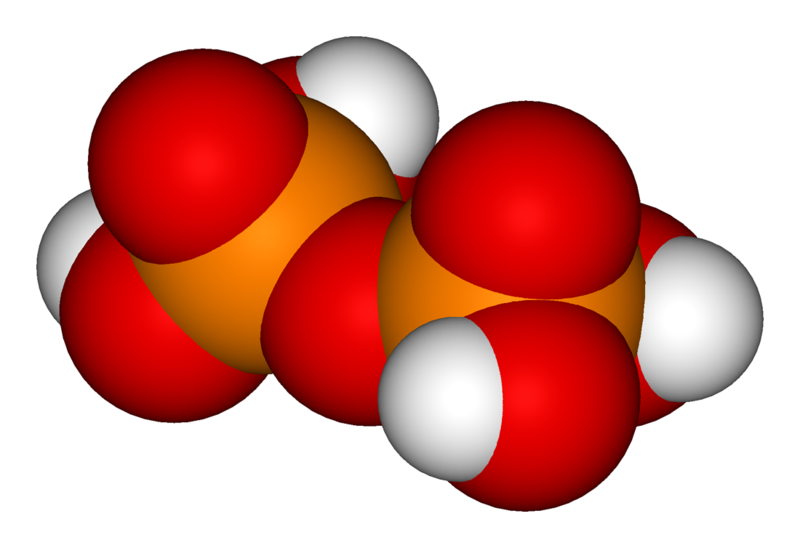
When two orthophosphoric acid molecules are condensed into one molecule, pyrophosphoric acid (H4P2O7) is obtained as follows:
- 2 H3PO4 → H4P2O7 + H2O
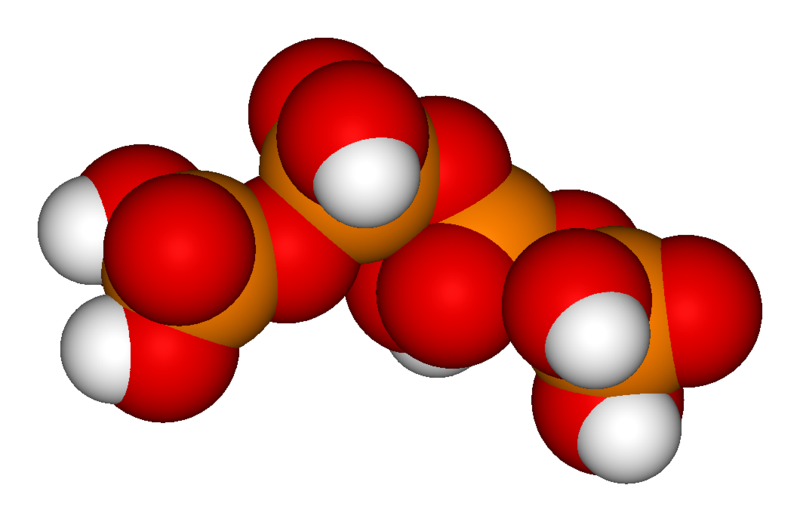
The chemical structure of pyrophosphoric acid is also shown in the illustration. There is also a separate article on Pyrophosphoric acid. Three orthophosphoric acid molecules can condense in a row to obtain tripolyphosphoric acid (H5P3O10), which is also shown in the illustration and has a separate article: Triphosphoric acid. This condensation process can continue with additional orthophosphoric acid units to obtain tetrapolyphosphoric acid (H6P4O13, pictured) and so on. Note that each extra phosphoric unit adds 1 extra H (hydrogen) atom, 1 extra P (phosphorus) atom, and 3 extra O (oxygen) atoms. The "backbone" chain of these types of molecules consists of alternating P and O atoms covalently bonded together. The term oligophosphoric acid refers to this series of phosphoric acids when a small number of phosphoric acids are joined, per the general formula Hn+2PnO3n+1, if n = 3, 4, 5, etc., oligo-.[2] Alternately, the above series follows the naming pattern of di- (pyrophosphoric), tri-, tetra-, penta-, hexaphosphoric acid, etc.
Oligophosphates
In a pyrophosphoric acid molecule, there are four hydrogens bonded to oxygens, and one, two, three, or all four can be lost as H+ ions. When all four are lost from pyrophosphoric acid, a pyrophosphate ion is formed. Because pyrophosphoric acids can undergo four dissociations, there are four Ka values for it, as well as four corresponding pKa values. Similarly, pyrophosphate is a base with four Kb and, of course, four pKb values for regaining the H+ ions in reverse order.
The situation with higher order oligophosphoric acids and oligophosphates continues in a similar way. Tripolyphosphoric acid can lose up to five H+ ions to form a tripolyphosphate ion, tetrapolyphosphoric acid can lose up to six H+ ions to form tetrapolyphosphate, etc. As more dissociations per molecule are possible, the intervals between individual pKa and pKb values now start becoming smaller on the pH scale.
Ortho-, pyro-, and tripolyphosphate compounds have been commonly used in detergents (i. e. cleaners) formulations. For example, see Sodium tripolyphosphate. Sometimes pyrophosphate, tripolyphosphate, tetrapolyphosphate, etc. are called diphosphate, triphosphate, tetraphosphate, etc., especially when they are part of phosphate esters in biochemistry.
Polyphosphoric acids
When n is very large, the polymer is polyphosphoric acid.[2] Polyphosphoric acid molecules can have dozens of such phosphoric units bonded in a row. A general formula for such poly-acid compounds is HO(PO2OH)xH, where x = number of phosphoric units in the molecule. The four oxygen atoms bonded to each phosphorus atom are in a tetrahedral configuration with the phosphorus in the center of the tetrahedron and the oxygens in each of the four corners.
Polyphosphates
As the polyphosphoric molecules grow increasingly larger and more complex, practically any number of the somewhat acidic -OH groups in them can dissociate to become negatively charged oxygens, forming numerous combinations of multiple-charged polyphosphoric/polyphosphate anions. Generally in an aqueous solution, the degree or percentage of dissociation depends on the pH of the solution.
Cyclo- or metaphosphoric acids and metaphosphates
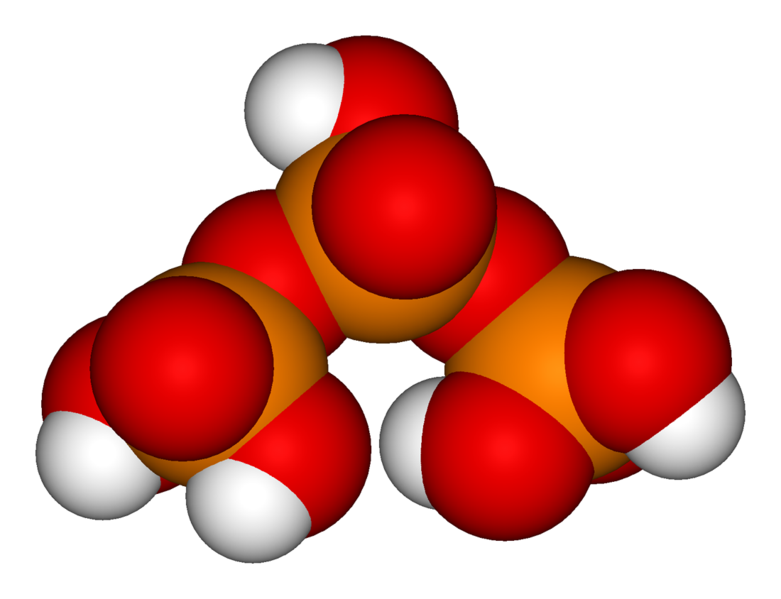
Phosphoric acid units can be bonded together in rings (cyclic structures) formed of metaphosphoric acid molecules. See metaphosphoric acid. The simplest such compound is trimetaphosphoric acid or cyclo-triphosphoric acid having the formula H3P3O9. Its structure is shown in the illustration. Since the ends are condensed, its formula has one less H2O (water) than tripolyphosphoric acid. A general formula for such cyclic compounds is (HPO3)x where x = number of phosphoric units in the molecule.
When these metaphosphoric acids lose their hydrogens as H+, cyclic anions called metaphosphates are formed. An example of a compound with such an anion is sodium hexametaphosphate (Na6P6O18) used as a sequestrant and a food additive.
Branched polyphosphates
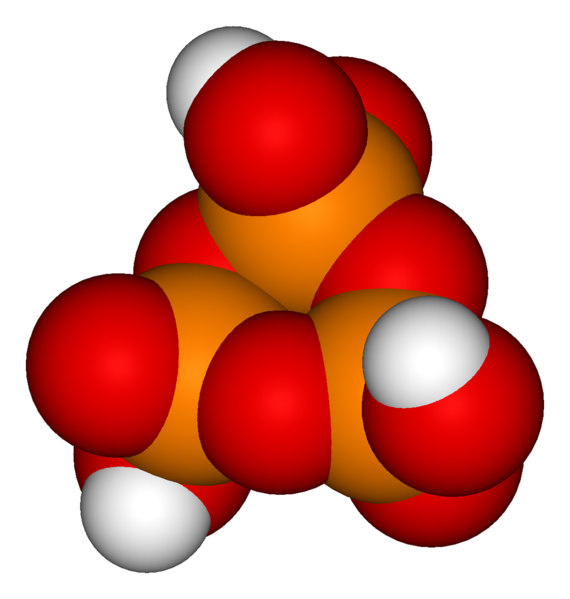
The third -OH group on an orthophosphoric acid unit can also be used for condensation with other phosphoric groups to form branches in the polyphosphoric/polyphosphate chains. The ultimate example of cyclic and branching condensation would be the cyclic four-phosphate unit double-branched to form the phosphoric anhydride P4O10; see illustration. Polyphosphoric acids that are also branched can be referred to as ultraphosphoric acid or ultrapolyphosphoric acid[3].
Paraphosphoric acid
Paraphosphoric acid (H5PO5) is obtained by burning phosphorus in dry air, or oxygen gas, appears in small crystals, like snow, and is formed, combined with water, by heating to redness pyrophosphoric and phosphoric acids; when fused, it cools into a brittle and transparent solid, resembling ice.[4] Paraphosphoric acid has phosphorus in the formal oxidation state of +5. It occurs as P(OH)5, CID 517655 with IUPAC name pentahydroxyphosphorane.
Thiophosphoric acid
Thiophosphoric acids have one or more oxygens replaced by sulfur. An example of a thiophosphoric acid ester (thiophosphorate) is on the page: Diazinon. Monothiophosphoric acid has the molecular formula H3PO3S, P(O)(OH)2(SH), is SID 50009326 and CID 167254 114 Da.[5] Dithiophosphoric acid has the molecular formula H3PO2S2, P(S)(OH)2(SH), is CAS number 15834-33-0, SID 729652 and CID 152119 130 Da.[6] A trithiophosphoric acid is phosphorotrithioic acid with the molecular formula H9C3OPS3, actually the methyl ester S,S,S,-trimethyl phophorotrithioate, H3CSP(=O)(SCH3)SCH3, CID 120321, IUPAC Name: bis(methylsulfanyl)phosphorylsulfanylmethane, 188 Da, CAS number 681-71-0.[7]
Perphosphoric acid
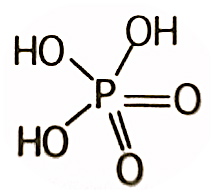
The use of per before the phosphoric indicates that the acid contains one more oxygen than phosphoric acid. For example, permonophosphoric acid has the formula H3PO5 instead of H3PO4 for orthophosphoric acid (monophosphoric acid).
Permonophosphoric acid is an acid formed when pyrophosphoric acid is treated with a large excess of hydrogen peroxide.
H4P2O7 + 2H2O2 <=> 2H3PO5 + H2O
Permonophosphoric acid 114 Da is a triprotic acid, yielding PO53- (permonophosphate ion) with its H+s removed. The preparation of permonophosphoric acid, H3PO5, was obtained by J. Schmidlin and P. Massini (Ber., 1910, 43, 1162).[8] Monoperphosphoric acid can be obtained when phosphorus pentoxide is treated with 30 % hydrogen peroxide, while cooled with ice-water.[9] But there are other forms of H3PO5.
When two oxygens are linked as in hydrogen peroxide, the permonophosphoric acid formed is peroxomonophosphoric acid (PMPA), (OH)2O=P-O-O-H, H3PO5. PMPA can be prepared by the perchloric acid hydrolysis of potassium peroxodiphosphate.[10] The species H3PO5, H2PO5-, HPO52-, and PO53- become predominant in this order with increasing pH and the electrophilic character of these species decreases.[10] PMPA exists as H2PO5- and HPO52- in the pH range 4-7.[10]
Monoperphosphoric acid and permonophosphoric acid are the same acid. Should the phosphoric acid be either an oligophosphoric acid or polyphosphoric acid as discussed below, the location of per may indicate different acids. For instance, perdiphosphoric acid would be H4P2O8, one more oxygen added to pyrophosphoric acid. But diperphosphoric acid could refer to two permonophosphoric acid molecules condensed with the loss of H2O to yield H4P2O9.
Perphosphates
Perphosphates have been produced from perphosphoric acid.[11]
Superphosphoric acid
Superphosphoric acid is a blend of orthophosphoric and polyphosphoric acid, CAS Number 8017-16-1. Superphosphoric acids can be in the concentration range of 62-85% P2O5 (86-117% H3PO4), but at about 90% H3PO4 the amount of condensed phosphate species becomes significant.[12] The concentration range of the superphosphoric acids is a range of increasing importance in fertilizer technology.[12]
Superphosphoric acid is sometimes called multiphosphoric acid or polyphosphoric acid.
Superphosphates
Superphosphates (salts or esters) can result from the use of superphosphoric acid. Superphosphate has been produced by acidulating phosphate rock with superphosphoric acid, where the superphosphoric acid contained about 49% of its phosphate as orthophosphate, 42% as pyrophosphate, and the remainder as polyphosphates.[13]
Hyperphosphoric acid
Hyperphosphoric acid is concentrated phosphoric acid. It can be prepared in two ways by:
(1) producing phosphorus pentoxide electrothermically and dissolving this oxide in a small quantity of water or
(2) concentrating phosphoric acid obtained by wet treatment with hot gas at temperatures above 700°C.[14]
It has also been called oleophosphoric acid.[14]
The first process produces hyperphosphoric acid of high purity and concentration but is very expensive. The second process produces a cheaper acid but introduces several impurities, among which are fluorine and sulphuric acid, and various metals, e.g. aluminium and iron. The metallic impurities exist as complexes in the form of polyphosphoric salts.[14]
Hyperphosphoric acid can be added to blood to release CO2.[15]
Hyperphosphates
Hyperphosphates (salts or esters) or oleophosphates can result from the use of hyperphosphoric acid. Hyperphosphate, when used to induce hyperphosphatemia, may directly promote calcium deposition and osteocalcin expression.[16]
Hydrolysis of polyphosphoric/polyphosphates
These phosphoric acids series are generally water-soluble considering the polarity of the molecules. Ammonium and alkali phosphates are also quite soluble in water. The alkaline earth salts start becoming less soluble and phosphate salts of various other metals are even less soluble. In aqueous solutions (solutions of water), water gradually (over the course of hours) hydrolyzes polyphosphates into smaller phosphates and finally into ortho-phosphate, given enough water. Higher temperature or acidic conditions can speed up the hydrolysis reactions considerably.
Conversely, polyphosphoric acids or polyphosphates are often formed by dehydrating a phosphoric acid solution; in other words, removing water from it often by heating and evaporating the water off.
Phosphate and phosphite esters

Here any R can be H
or some other organic radical
The -OH groups in phosphoric acids can also condense with the hydroxyl groups of alcohols to form phosphate esters. Since orthophosphoric acid has three -OH groups, it can esterify with one, two, or three alcohol molecules to form a mono-, di-, or triester. See the general structure image of an ortho- (or mono-) phosphate ester below on the left, where any of the R groups can be a hydrogen or an organic radical. Pyro- (or di-) phosphate esters and tripoly- (or tri-) phosphate esters, etc. are also possible. Any -OH groups on the phosphates in these ester molecules may lose H+ ions to form anions, again depending on the pH in a solution. In the biochemistry of living organisms, there are many kinds of (mono)phosphate, diphosphate, and triphosphate compounds (essentially esters), many of which play a significant role in metabolism such as adenosine diphosphate (ADP) and triphosphate (ATP).

Here any R can be H
or some other organic radical
Similarly, phosphorous acid can bond with alcohol molecules to form a phosphite ester. See the general structure image below on the right. The two dots on the P represent the lone electron pair of the phosphorus atom.
References
- ↑ Robertson DS (2004). "A possible natural antibacterial compound in the human metabolism". Med Hypotheses. 63 (3): 554–5. PMID 15288387.
- ↑ 2.0 2.1 Wiberg E, Wiberg N, Holleman AF (2001). Inorganic chemistry. Academic Press. p. 727-8. ISBN 0123526515, 9780123526519 Check
|isbn=value: invalid character (help). More than one of|pages=and|page=specified (help) - ↑ Shiba T, Shiba T, Yamaoka M, Uematsu T, Takahashi Y, Tanaka H, Kogo T, Shindo M. "Antiinflammtory agent and antiinflammatory medical material". Unknown parameter
|USPTO Application #=ignored (help) - ↑ Alonzo Gray (2008). Elements of Chemistry. BiblioBazaar, LLC. p. 205. ISBN 0559049102, 9780559049101 Check
|isbn=value: invalid character (help). More than one of|pages=and|page=specified (help) - ↑ "thiophosphoric acid - Substance Summary - PubChem Public Chemical Database".
- ↑ "phosphorodithioic acid - Compound Summary - PubChem Public Chemical Database".
- ↑ "S,S,S-trimethyl phosphorotrithioate - Compound Summary - PubChem Public Chemical Database".
- ↑ Ogata Y, Urasaki I, Nagura K, Satomi N (1974). "Kinetics of the aromatic hydroxylation with permonophosphoric acid". Tetrahedron. 30 (17): 3021–5. doi:10.1016/S0040-4020(01)97547-7 .
- ↑ Joseph William Mellor (1912). Modern Inorganic Chemistry. Longmans, Green. p. 597. More than one of
|pages=and|page=specified (help) - ↑ 10.0 10.1 10.2 Panigrahi GP, Panda R (1980). "Oxidation studies with peroxomonophosphoric acid. III. A kinetic and mechanistic study of oxidation of dialkyl sulfides". Bull Chem Soc Jpn. 53 (8): 2366–70.
- ↑ Jones NC (1929). "The anode reactions of fluorine". J Phys Chem. 33 (6): 801–24. doi:10.1021/j150300a001. Unknown parameter
|month=ignored (help) - ↑ 12.0 12.1 Luff BB, Reed RB, Wakefield ZT (1971). "Enthalpy of dilution of superphosphoric acids". J Chem Eng Data. 16 (3): 342–4. doi:10.1021/je60050a033. Unknown parameter
|month=ignored (help) - ↑ Phillips AB, Young RD, Heil FG, Norton MM (1960). "Fertilizer technology, high-analysis superphosphate by the reaction of phosphate rock with superphosphoric acid". J Agric Food Chem. 8 (4): 310–5. doi:10.1021/jf60110a016. Unknown parameter
|month=ignored (help) - ↑ 14.0 14.1 14.2 "Process for obtaining concentrated phosphoric acid".
- ↑ Sidossis LS, Mittendorfer B, Chinkes D, Walser E, Wolfe RR (1999). "Effect of hyperglycemia-hyperinsulinemia on whole body and regional fatty acid metabolism". Am J Physiol. 276 ((3 Pt 1)): E427–34. PMID 10070006. Unknown parameter
|month=ignored (help) - ↑ Lu W, Wang X, Zhao X (2007). "Study of vascular smooth muscle cell calcification induced by hyperphosphate and intervented by phosphonoformic acid". J Nanjing Med Univ. 21 (6): 377–81. doi:10.1016/S1007-4376(07)60082-3. Unknown parameter
|month=ignored (help)