Oxacillin: Difference between revisions
m (Protected "Oxacillin": Protecting pages from unwanted edits ([edit=sysop] (indefinite) [move=sysop] (indefinite))) |
m (Protected "Oxacillin": Bot: Protecting all pages from category Drug ([Edit=Allow only administrators] (indefinite) [Move=Allow only administrators] (indefinite))) |
||
| (27 intermediate revisions by 4 users not shown) | |||
| Line 1: | Line 1: | ||
{{ | {{DrugProjectFormSinglePage | ||
|IUPAC_name=(2'' | |authorTag={{Ammu}} | ||
|image=Oxacillin skeletal. | |genericName=Oxacillin | ||
|CAS_number=66-79-5 | |aOrAn=an | ||
|ATC_prefix=J01 | |drugClass=[[antibiotic]] | ||
|ATC_suffix= | |indicationType=treatment | ||
|ATC_supplemental={{ | |indication=[[infections]] caused by penicillinase producing [[staphylococci]] | ||
|PubChem=6196 | |adverseReactions=[[rash]], [[diarrhea]], [[nausea]] | ||
|DrugBank= | |blackBoxWarningTitle=<span style="color:#FF0000;">ConditionName: </span> | ||
|C=19|H=19|N=3|O=5|S=1 | |blackBoxWarningBody=<i><span style="color:#FF0000;">ConditionName: </span></i> | ||
|molecular_weight=401. | |||
| | * Content | ||
| | |||
| | <!--Adult Indications and Dosage--> | ||
| | |||
| | <!--FDA-Labeled Indications and Dosage (Adult)--> | ||
| | |fdaLIADAdult=* Oxacillin is indicated in the treatment of infections caused by penicillinase producing [[staphylococci]] which have demonstrated susceptibility to the drug. | ||
| | * Cultures and susceptibility tests should be performed initially to determine the causative organism and its susceptibility to the drug. | ||
| | * Oxacillin may be used to initiate therapy in suspected cases of resistant [[staphylococcal infections]] prior to the availability of susceptibility test results. Oxacillin should not be used in infections caused by organisms susceptible to [[penicillin G]]. If the susceptibility tests indicate that the [[infection]] is due to an organism other than a resistant [[Staphylococcus]], therapy should not be continued with oxacillin. | ||
| | * To reduce the development of drug-resistant [[bacteria]] and maintain the effectiveness of Oxacillin Injection, USP and other antibacterial drugs, Oxacillin Injection, USP should be used only to treat or prevent infections that are proven or strongly suspected to be caused by susceptible bacteria. When culture and susceptibility information are available, they should be considered in selecting or modifying antibacterial therapy. In the absence of such data, local epidemiology and susceptibility patterns may contribute to the empiric selection of therapy. | ||
| | |offLabelAdultGuideSupport=There is limited information regarding <i>Off-Label Guideline-Supported Use</i> of {{PAGENAME}} in adult patients. | ||
| | <!--Non–Guideline-Supported Use (Adult)--> | ||
| | |offLabelAdultNoGuideSupport=* [[Bacteremia]] associated with intravascular line, due to methicillin-susceptible [[Staphylococcus aureus]]. | ||
* Bacterial [[meningitis]], methicillin-susceptible [[Staphylococcus aureus]]. | |||
* Complication of [[catheter]]; Prophylaxis - Infectious disease; Prophylaxis. | |||
* Infection of [[bone]] - Infectious disorder. | |||
|fdaLIADPed=There is limited information regarding <i>FDA-Labeled Use</i> of {{PAGENAME}} in pediatric patients. | |||
<!--Off-Label Use and Dosage (Pediatric)--> | |||
<!--Guideline-Supported Use (Pediatric)--> | |||
|offLabelPedGuideSupport=There is limited information regarding <i>Off-Label Guideline-Supported Use</i> of {{PAGENAME}} in pediatric patients. | |||
<!--Non–Guideline-Supported Use (Pediatric)--> | |||
|offLabelPedNoGuideSupport=There is limited information regarding <i>Off-Label Non–Guideline-Supported Use</i> of {{PAGENAME}} in pediatric patients. | |||
<!--Contraindications--> | |||
|contraindications=* A history of a [[hypersensitivity]] (anaphylactic) reaction to any penicillin is a contraindication. Solutions containing dextrose may be contraindicated in patients with known allergy to corn or corn products. | |||
|warnings=* Serious and occasionally fatal [[hypersensitivity]] (anaphylactic shock with collapse) reactions have occurred in patients receiving [[penicillin]]. | |||
* The incidence of anaphylactic shock in all penicillin-treated patients is between 0.015 and 0.04 percent. Anaphylactic shock resulting in death has occurred in approximately 0.002 percent of the patients treated. Although anaphylaxis is more frequent following parenteral administration, it has occurred in patients receiving oral penicillins. | |||
* When penicillin therapy is indicated, it should be initiated only after a comprehensive patient drug and allergy history has been obtained. If an allergic reaction occurs, the drug should be discontinued and the patient should receive supportive treatment, e.g., artificial maintenance of ventilation, pressor amines, antihistamines, and [[corticosteroid]]s. Individuals with a history of penicillin hypersensitivity may also experience allergic reactions when treated with a [[cephalosporin]]. | |||
* [[Clostridium difficile associated diarrhea]] (CDAD) has been reported with use of nearly all antibacterial agents, including Oxacillin Injection, USP, and may range in severity from mild [[diarrhea]] to fatal [[colitis]]. Treatment with antibacterial agents alters the normal flora of the colon leading to overgrowth of C. difficile. | |||
* C. difficile produces toxins A and B which contribute to the development of CDAD. Hypertoxin producing strains of C. difficile cause increased morbidity and mortality, as these infections can be refractory to antimicrobial therapy and may require colectomy. CDAD must be considered in all patients who present with diarrhea following antibiotic use. | |||
* Careful medical history is necessary since CDAD has been reported to occur over two months after the administration of antibacterial agents. | |||
* If CDAD is suspected or confirmed, ongoing antibiotic use not directed against C. difficile may need to be discontinued. Appropriate fluid and electrolyte management, protein supplementation, antibiotic treatment of C. difficile, and surgical evaluation should be instituted as clinically indicated. | |||
|clinicalTrials=There is limited information regarding <i>Clinical Trial Experience</i> of {{PAGENAME}} in the drug label. | |||
|postmarketing=* The reported incidence of allergic reactions to penicillin ranges from 0.7 to 10 percent. Sensitization is usually the result of treatment but some individuals have had immediate reactions when first treated. | |||
* In such cases, it is thought that the patients may have had prior exposure to the drug via trace amounts present in milk and vaccines. | |||
* Two types of allergic reactions to penicillins are noted clinically, immediate and delayed. | |||
* Immediate reactions usually occur within 20 minutes of administration and range in severity from urticaria and pruritus to angioneurotic edema, [[laryngospasm]], [[bronchospasm]], [[hypotension]], vascular collapse and death. Such immediate anaphylactic reactions are very rare and usually occur after parenteral therapy but have occurred in patients receiving oral therapy. | |||
* Another type of immediate reaction, an accelerated reaction, may occur between 20 minutes and 48 hours after administration and may include [[urticaria]], [[pruritus]], and [[fever]]. Although laryngeal edema, [[laryngospasm]], and [[hypotension]] occasionally occur, fatality is uncommon. Delayed allergic reactions to penicillin therapy usually occur after 48 hours and sometimes as late as 2 to 4 weeks after initiation of therapy. | |||
* Manifestations of this type of reaction include serum sickness-like symptoms (i.e., [[fever]], malaise, [[urticaria]], [[myalgia]], [[arthralgia]], [[abdominal pain]]) and various skin rashes. [[Nausea]], [[vomiting]], [[diarrhea]], [[stomatitis]], black or hairy [[tongue]], and other symptoms of gastrointestinal irritation may occur, especially during oral penicillin therapy. | |||
=====Nervous System Reactions===== | |||
* Neurotoxic reactions similar to those observed with penicillin G may occur with large intravenous doses of oxacillin, especially with patients with renal insufficiency. | |||
=====Urogenital Reactions===== | |||
* Renal tubular damage and interstitial [[nephritis]] have been associated infrequently with the administration of oxacillin. Manifestations of this reaction may include [[rash]], [[fever]], [[eosinophilia]], [[hematuria]], [[proteinuria]], and renal insufficiency. | |||
=====Gastrointestinal Reactions===== | |||
* Pseudomembranous colitis has been reported with the use of oxacillin. The onset of [[pseudomembranous colitis]] symptoms may occur during or after antibiotic treatment. | |||
=====Metabolic Reactions===== | |||
* Hepatotoxicity, characterized by [[fever]], [[nausea]], and [[vomiting]] associated with abnormal liver function tests, mainly elevated SGOT levels, has been associated with the use of oxacillin. | |||
|drugInteractions=* Tetracycline, a bacteriostatic antibiotic, may antagonize the bactericidal effect of penicillin and concurrent use of these drugs should be avoided. | |||
* Oxacillin blood levels may be prolonged by concurrent administration of [[probenecid]] which blocks the renal tubular secretion of penicillins. | |||
|FDAPregCat=B | |||
|useInPregnancyFDA=* Reproduction studies performed in the mouse, rat, and rabbit have revealed no evidence of impaired fertility or harm to the fetus due to the penicillinase-resistant penicillins. | |||
* Human experience with the penicillins during [[pregnancy]] has not shown any positive evidence of adverse effects on the fetus. There are, however, no adequate or well-controlled studies in pregnant women showing conclusively that harmful effects of these drugs on the fetus can be excluded. Because animal reproduction studies are not always predictive of human response, this drug should be used during pregnancy only if clearly needed. | |||
|useInPregnancyAUS=* '''Australian Drug Evaluation Committee (ADEC) Pregnancy Category''' | |||
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of {{PAGENAME}} in women who are pregnant. | |||
|useInLaborDelivery=There is no FDA guidance on use of {{PAGENAME}} during labor and delivery. | |||
|useInNursing=* Penicillins are excreted in human milk. Caution should be exercised when penicillins are administered to a nursing woman. | |||
|useInPed=* Because of incompletely developed renal function in pediatric patients, oxacillin may not be completely excreted, with abnormally high blood levels resulting. | |||
* Frequent blood levels are advisable in this group with dosage adjustments when necessary. All pediatric patients treated with penicillins should be monitored closely for clinical and laboratory evidence of toxic or adverse effects. Safety and effectiveness in pediatric patients have not been established. | |||
* The potential for toxic effects in pediatric patients from chemicals that may leach from the single dose premixed intravenous preparation in plastic containers has not been evaluated. | |||
|useInGeri=* Clinical studies of Oxacillin injection did not include sufficient number of subjects aged 65 and over to determine whether they respond differently from younger subjects. | |||
* Other reported clinical experience has not identified differences in responses between the elderly and younger patients. In general, dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy. | |||
* This drug is known to be substantially excreted by the kidney, and the risk of toxic reactions to this drug may be greater in patients with impaired renal function. Because elderly patients are more likely to have decreased renal function, care should be taken in dose selection, and it may be useful to monitor renal function. | |||
* Oxacillin Injection contains 92.4 mg (4.02 mEq) of sodium per gram. At the usual recommended doses, patients would receive between 92.4 and 554 mg/day (4.02 and 24.1 mEq) of sodium. The geriatric population may respond with a blunted natriuresis to salt loading. This may be clinically important with regard to such diseases as congestive heart failure. | |||
|useInGender=There is no FDA guidance on the use of {{PAGENAME}} with respect to specific gender populations. | |||
|useInRace=There is no FDA guidance on the use of {{PAGENAME}} with respect to specific racial populations. | |||
|useInRenalImpair=There is no FDA guidance on the use of {{PAGENAME}} in patients with renal impairment. | |||
|useInHepaticImpair=There is no FDA guidance on the use of {{PAGENAME}} in patients with hepatic impairment. | |||
|useInReproPotential=There is no FDA guidance on the use of {{PAGENAME}} in women of reproductive potentials and males. | |||
|useInImmunocomp=There is no FDA guidance one the use of {{PAGENAME}} in patients who are immunocompromised. | |||
<!--Administration and Monitoring--> | |||
|administration=* [[Oral]]. | |||
* [[Intravenous]]. | |||
|monitoring=* Because elderly patients are more likely to have decreased renal function, care should be taken in dose selection, and it may be useful to monitor renal function. | |||
* If any impairment of renal function is suspected or known to exist, a reduction in the total dosage should be considered and blood levels monitored to avoid possible neurotoxic reactions. | |||
* AST (SGOT) and ALT (SGPT) values should be obtained periodically during therapy to monitor for possible liver function abnormalities. | |||
* All pediatric patients treated with penicillins should be monitored closely for clinical and laboratory evidence of toxic or adverse effects. Safety and effectiveness in pediatric patients have not been established. | |||
<!--IV Compatibility--> | |||
|IVCompat=There is limited information regarding <i>IV Compatibility</i> of {{PAGENAME}} in the drug label. | |||
<!--Overdosage--> | |||
|overdose=* The signs and symptoms of oxacillin overdosage are those described in the ADVERSE REACTIONS section. If signs or symptoms occur, discontinue use of the medication, treat symptomatically, and institute appropriate supportive measures. | |||
|drugBox={{Drugbox2 | |||
| Verifiedfields = changed | |||
| Watchedfields = changed | |||
| verifiedrevid = 408783354 | |||
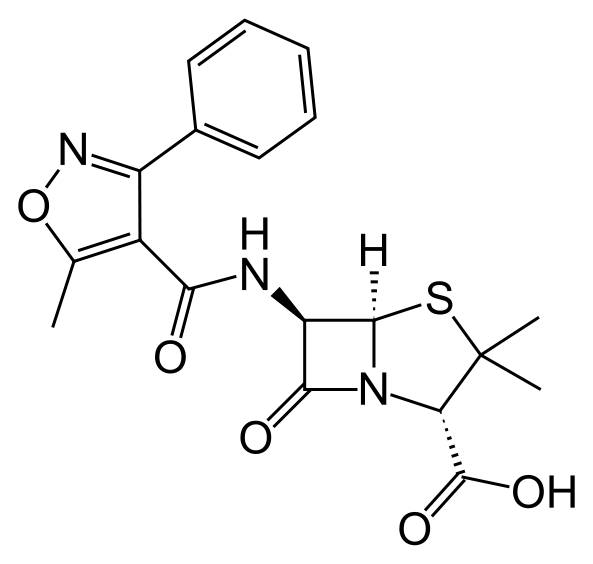
| IUPAC_name = (2''S'',5''R'',6''R'')-3,3-dimethyl-6-[(5-methyl-3-phenyl-<br>1,2-oxazole-4-carbonyl)amino]-7-oxo-4-thia-1-<br>azabicyclo[3.2.0]heptane-2-carboxylic acid | |||
| image = Oxacillin skeletal.png | |||
| image2 = | |||
<!--Clinical data--> | |||
| tradename = Bactocill | |||
| Drugs.com = {{drugs.com|monograph|bactocill}} | |||
| MedlinePlus = a685020 | |||
| pregnancy_AU = <!-- A / B1 / B2 / B3 / C / D / X --> | |||
| pregnancy_US = <!-- A / B / C / D / X --> | |||
| legal_AU = <!-- Unscheduled / S2 / S4 / S8 --> | |||
| legal_UK = <!-- GSL / P / POM / CD --> | |||
| legal_US = <!-- OTC / Rx-only --> | |||
<!--Identifiers--> | |||
| CASNo_Ref = {{cascite|correct|CAS}} | |||
| CAS_number_Ref = {{cascite|correct|??}} | |||
| CAS_number = 66-79-5 | |||
| ATC_prefix = J01 | |||
| ATC_suffix = CF04 | |||
| ATC_supplemental = {{ATCvet|J51|CF04}} | |||
| PubChem = 6196 | |||
| DrugBank_Ref = {{drugbankcite|changed|drugbank}} | |||
| DrugBank = DB00713 | |||
| ChemSpiderID_Ref = {{chemspidercite|changed|chemspider}} | |||
| ChemSpiderID = 5961 | |||
| UNII_Ref = {{fdacite|correct|FDA}} | |||
| UNII = UH95VD7V76 | |||
| KEGG_Ref = {{keggcite|correct|kegg}} | |||
| KEGG = D08307 | |||
| ChEBI_Ref = {{ebicite|changed|EBI}} | |||
| ChEBI = 49566 | |||
| ChEMBL_Ref = {{ebicite|changed|EBI}} | |||
| ChEMBL = 819 | |||
<!--Chemical data--> | |||
| C=19 | H=19 | N=3 | O=5 | S=1 | |||
| molecular_weight = 401.436 g/mol | |||
| smiles = Cc1c(c(no1)c2ccccc2)/C(=N/[C@H]3[C@@H]4N(C3=O)[C@H](C(S4)(C)C)C(=O)O)/O | |||
| InChI = 1S/C19H18ClN3O5S/c1-8-11(12(22-28-8)9-6-4-5-7-10(9)20)15(24)21-13-16(25)23-14(18(26)27)19(2,3)29-17(13)23/h4-7,13-14,17H,1-3H3,(H,21,24)(H,26,27)/t13-,14+,17-/m1/s1 | |||
| InChIKey = LQOLIRLGBULYKD-JKIFEVAISA-N | |||
| StdInChI_Ref = {{stdinchicite|changed|chemspider}} | |||
| StdInChI = 1S/C19H19N3O5S/c1-9-11(12(21-27-9)10-7-5-4-6-8-10)15(23)20-13-16(24)22-14(18(25)26)19(2,3)28-17(13)22/h4-8,13-14,17H,1-3H3,(H,20,23)(H,25,26)/t13-,14+,17-/m1/s1 | |||
| StdInChIKey_Ref = {{stdinchicite|changed|chemspider}} | |||
| StdInChIKey = UWYHMGVUTGAWSP-JKIFEVAISA-N | |||
| density = 1.49 | |||
| boiling_point = 686.8 | |||
}} | |||
|mechAction=* Penicillinase-resistant penicillins exert a bactericidal action against penicillin susceptible microorganisms during the state of active multiplication. All penicillins inhibit the biosynthesis of the bacterial cell wall. | |||
<!--Structure--> | |||
|structure=* Oxacillin Injection, USP is a sterile injectable product containing oxacillin which is added as oxacillin sodium, a semisynthetic penicillin derived from the penicillin nucleus, 6-aminopenicillanic acid. | |||
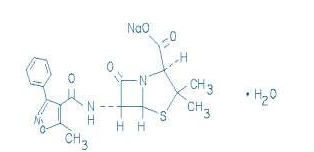
* The structural formula of oxacillin sodium is as follows: | |||
: [[File:Oxacillin 1.png|thumb|none|600px|This image is provided by the National Library of Medicine.]] | |||
* Oxacillin Injection, USP is a frozen, iso-osmotic, sterile, nonpyrogenic premixed 50 mL solution containing 1 g or 2 g of oxacillin added as oxacillin sodium. Dextrose, USP has been added to the above dosages to adjust osmolality (approximately 1.5 g and 300 mg as dextrose hydrous to the 1 g and 2 g dosages respectively). | |||
* Sodium Citrate Hydrous, USP has been added as a buffer (approximately 150 mg and 300 mg to the 1 g and 2 g dosages, respectively). The pH has been adjusted with hydrochloric acid and may have been adjusted with sodium hydroxide. The pH is 6.5 (6.0 to 8.5). The solution is intended for intravenous use after thawing to room temperature. | |||
* This GALAXY container (PL 2040) is fabricated from a specially designed multilayer plastic (PL 2040). Solutions are in contact with the polyethylene layer of this container and can leach out certain chemical components of the plastic in very small amounts within the expiration period. The suitability of the plastic has been confirmed in tests in animals according to the USP biological tests for plastic containers, as well as by tissue culture toxicity studies. | |||
<!--Pharmacodynamics--> | |||
|PD=There is limited information regarding <i>Pharmacodynamics</i> of {{PAGENAME}} in the drug label. | |||
<!--Pharmacokinetics--> | |||
|PK=There is limited information regarding <i>Pharmacokinetics</i> of {{PAGENAME}} in the drug label. | |||
<!--Nonclinical Toxicology--> | |||
|nonClinToxic=There is limited information regarding <i>Nonclinical Toxicology</i> of {{PAGENAME}} in the drug label. | |||
<!--Clinical Studies--> | |||
|howSupplied=*Oxacillin Injection, USP is supplied as a premixed frozen iso-osmotic solution in 50 mL single dose GALAXY plastic containers as follows: | |||
* 2G3538 NDC 0338-1013-41 1 gram oxacillin | |||
* 2G3539 NDC 0338-1015-41 2 grams oxacillin | |||
|storage=* Store at or below -20°C/-4°F | |||
|packLabel=<!--Patient Counseling Information--> | |||
|fdaPatientInfo=There is limited information regarding <i>Patient Counseling Information</i> of {{PAGENAME}} in the drug label. | |||
<!--Precautions with Alcohol--> | |||
|alcohol=* Alcohol-{{PAGENAME}} interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication. | |||
<!--Brand Names--> | |||
|brandNames=* OXACILLIN®<ref>{{Cite web | title = OXACILLIN- oxacillin sodium injection, solution | url =http://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=d893e45b-7f18-4c67-b10c-1e2e52a6b3ef }}</ref> | |||
<!--Look-Alike Drug Names--> | |||
|drugShortage= | |||
}} | |||
{{LabelImage | |||
|fileName=Ox02.jpg | |||
}} | |||
{{LabelImage | |||
|fileName=Ox03.jpg | |||
}} | }} | ||
{{ | {{LabelImage | ||
|fileName=DailyMed - OXACILLIN- oxacillin sodium injection, solution .png | |||
}} | |||
<!--Pill Image--> | |||
<!--Label Display Image--> | |||
<!--Category--> | |||
[[Category:Drug]] | |||
[[Category:Beta-lactam antibiotics]] | [[Category:Beta-lactam antibiotics]] | ||
[[Category:Isoxazoles]] | |||
[[ | |||
Latest revision as of 16:51, 20 August 2015
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Ammu Susheela, M.D. [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Oxacillin is an antibiotic that is FDA approved for the treatment of infections caused by penicillinase producing staphylococci. Common adverse reactions include rash, diarrhea, nausea.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
- Oxacillin is indicated in the treatment of infections caused by penicillinase producing staphylococci which have demonstrated susceptibility to the drug.
- Cultures and susceptibility tests should be performed initially to determine the causative organism and its susceptibility to the drug.
- Oxacillin may be used to initiate therapy in suspected cases of resistant staphylococcal infections prior to the availability of susceptibility test results. Oxacillin should not be used in infections caused by organisms susceptible to penicillin G. If the susceptibility tests indicate that the infection is due to an organism other than a resistant Staphylococcus, therapy should not be continued with oxacillin.
- To reduce the development of drug-resistant bacteria and maintain the effectiveness of Oxacillin Injection, USP and other antibacterial drugs, Oxacillin Injection, USP should be used only to treat or prevent infections that are proven or strongly suspected to be caused by susceptible bacteria. When culture and susceptibility information are available, they should be considered in selecting or modifying antibacterial therapy. In the absence of such data, local epidemiology and susceptibility patterns may contribute to the empiric selection of therapy.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Oxacillin in adult patients.
Non–Guideline-Supported Use
- Bacteremia associated with intravascular line, due to methicillin-susceptible Staphylococcus aureus.
- Bacterial meningitis, methicillin-susceptible Staphylococcus aureus.
- Complication of catheter; Prophylaxis - Infectious disease; Prophylaxis.
- Infection of bone - Infectious disorder.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
There is limited information regarding FDA-Labeled Use of Oxacillin in pediatric patients.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Oxacillin in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Oxacillin in pediatric patients.
Contraindications
- A history of a hypersensitivity (anaphylactic) reaction to any penicillin is a contraindication. Solutions containing dextrose may be contraindicated in patients with known allergy to corn or corn products.
Warnings
- Serious and occasionally fatal hypersensitivity (anaphylactic shock with collapse) reactions have occurred in patients receiving penicillin.
- The incidence of anaphylactic shock in all penicillin-treated patients is between 0.015 and 0.04 percent. Anaphylactic shock resulting in death has occurred in approximately 0.002 percent of the patients treated. Although anaphylaxis is more frequent following parenteral administration, it has occurred in patients receiving oral penicillins.
- When penicillin therapy is indicated, it should be initiated only after a comprehensive patient drug and allergy history has been obtained. If an allergic reaction occurs, the drug should be discontinued and the patient should receive supportive treatment, e.g., artificial maintenance of ventilation, pressor amines, antihistamines, and corticosteroids. Individuals with a history of penicillin hypersensitivity may also experience allergic reactions when treated with a cephalosporin.
- Clostridium difficile associated diarrhea (CDAD) has been reported with use of nearly all antibacterial agents, including Oxacillin Injection, USP, and may range in severity from mild diarrhea to fatal colitis. Treatment with antibacterial agents alters the normal flora of the colon leading to overgrowth of C. difficile.
- C. difficile produces toxins A and B which contribute to the development of CDAD. Hypertoxin producing strains of C. difficile cause increased morbidity and mortality, as these infections can be refractory to antimicrobial therapy and may require colectomy. CDAD must be considered in all patients who present with diarrhea following antibiotic use.
- Careful medical history is necessary since CDAD has been reported to occur over two months after the administration of antibacterial agents.
- If CDAD is suspected or confirmed, ongoing antibiotic use not directed against C. difficile may need to be discontinued. Appropriate fluid and electrolyte management, protein supplementation, antibiotic treatment of C. difficile, and surgical evaluation should be instituted as clinically indicated.
Adverse Reactions
Clinical Trials Experience
There is limited information regarding Clinical Trial Experience of Oxacillin in the drug label.
Postmarketing Experience
- The reported incidence of allergic reactions to penicillin ranges from 0.7 to 10 percent. Sensitization is usually the result of treatment but some individuals have had immediate reactions when first treated.
- In such cases, it is thought that the patients may have had prior exposure to the drug via trace amounts present in milk and vaccines.
- Two types of allergic reactions to penicillins are noted clinically, immediate and delayed.
- Immediate reactions usually occur within 20 minutes of administration and range in severity from urticaria and pruritus to angioneurotic edema, laryngospasm, bronchospasm, hypotension, vascular collapse and death. Such immediate anaphylactic reactions are very rare and usually occur after parenteral therapy but have occurred in patients receiving oral therapy.
- Another type of immediate reaction, an accelerated reaction, may occur between 20 minutes and 48 hours after administration and may include urticaria, pruritus, and fever. Although laryngeal edema, laryngospasm, and hypotension occasionally occur, fatality is uncommon. Delayed allergic reactions to penicillin therapy usually occur after 48 hours and sometimes as late as 2 to 4 weeks after initiation of therapy.
- Manifestations of this type of reaction include serum sickness-like symptoms (i.e., fever, malaise, urticaria, myalgia, arthralgia, abdominal pain) and various skin rashes. Nausea, vomiting, diarrhea, stomatitis, black or hairy tongue, and other symptoms of gastrointestinal irritation may occur, especially during oral penicillin therapy.
Nervous System Reactions
- Neurotoxic reactions similar to those observed with penicillin G may occur with large intravenous doses of oxacillin, especially with patients with renal insufficiency.
Urogenital Reactions
- Renal tubular damage and interstitial nephritis have been associated infrequently with the administration of oxacillin. Manifestations of this reaction may include rash, fever, eosinophilia, hematuria, proteinuria, and renal insufficiency.
Gastrointestinal Reactions
- Pseudomembranous colitis has been reported with the use of oxacillin. The onset of pseudomembranous colitis symptoms may occur during or after antibiotic treatment.
Metabolic Reactions
- Hepatotoxicity, characterized by fever, nausea, and vomiting associated with abnormal liver function tests, mainly elevated SGOT levels, has been associated with the use of oxacillin.
Drug Interactions
- Tetracycline, a bacteriostatic antibiotic, may antagonize the bactericidal effect of penicillin and concurrent use of these drugs should be avoided.
- Oxacillin blood levels may be prolonged by concurrent administration of probenecid which blocks the renal tubular secretion of penicillins.
Use in Specific Populations
Pregnancy
- Reproduction studies performed in the mouse, rat, and rabbit have revealed no evidence of impaired fertility or harm to the fetus due to the penicillinase-resistant penicillins.
- Human experience with the penicillins during pregnancy has not shown any positive evidence of adverse effects on the fetus. There are, however, no adequate or well-controlled studies in pregnant women showing conclusively that harmful effects of these drugs on the fetus can be excluded. Because animal reproduction studies are not always predictive of human response, this drug should be used during pregnancy only if clearly needed.
- Australian Drug Evaluation Committee (ADEC) Pregnancy Category
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Oxacillin in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Oxacillin during labor and delivery.
Nursing Mothers
- Penicillins are excreted in human milk. Caution should be exercised when penicillins are administered to a nursing woman.
Pediatric Use
- Because of incompletely developed renal function in pediatric patients, oxacillin may not be completely excreted, with abnormally high blood levels resulting.
- Frequent blood levels are advisable in this group with dosage adjustments when necessary. All pediatric patients treated with penicillins should be monitored closely for clinical and laboratory evidence of toxic or adverse effects. Safety and effectiveness in pediatric patients have not been established.
- The potential for toxic effects in pediatric patients from chemicals that may leach from the single dose premixed intravenous preparation in plastic containers has not been evaluated.
Geriatic Use
- Clinical studies of Oxacillin injection did not include sufficient number of subjects aged 65 and over to determine whether they respond differently from younger subjects.
- Other reported clinical experience has not identified differences in responses between the elderly and younger patients. In general, dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy.
- This drug is known to be substantially excreted by the kidney, and the risk of toxic reactions to this drug may be greater in patients with impaired renal function. Because elderly patients are more likely to have decreased renal function, care should be taken in dose selection, and it may be useful to monitor renal function.
- Oxacillin Injection contains 92.4 mg (4.02 mEq) of sodium per gram. At the usual recommended doses, patients would receive between 92.4 and 554 mg/day (4.02 and 24.1 mEq) of sodium. The geriatric population may respond with a blunted natriuresis to salt loading. This may be clinically important with regard to such diseases as congestive heart failure.
Gender
There is no FDA guidance on the use of Oxacillin with respect to specific gender populations.
Race
There is no FDA guidance on the use of Oxacillin with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Oxacillin in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Oxacillin in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Oxacillin in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Oxacillin in patients who are immunocompromised.
Administration and Monitoring
Administration
- Oral.
Monitoring
- Because elderly patients are more likely to have decreased renal function, care should be taken in dose selection, and it may be useful to monitor renal function.
- If any impairment of renal function is suspected or known to exist, a reduction in the total dosage should be considered and blood levels monitored to avoid possible neurotoxic reactions.
- AST (SGOT) and ALT (SGPT) values should be obtained periodically during therapy to monitor for possible liver function abnormalities.
- All pediatric patients treated with penicillins should be monitored closely for clinical and laboratory evidence of toxic or adverse effects. Safety and effectiveness in pediatric patients have not been established.
IV Compatibility
There is limited information regarding IV Compatibility of Oxacillin in the drug label.
Overdosage
- The signs and symptoms of oxacillin overdosage are those described in the ADVERSE REACTIONS section. If signs or symptoms occur, discontinue use of the medication, treat symptomatically, and institute appropriate supportive measures.
Pharmacology
| |
Oxacillin
| |
| Systematic (IUPAC) name | |
| (2S,5R,6R)-3,3-dimethyl-6-[(5-methyl-3-phenyl- 1,2-oxazole-4-carbonyl)amino]-7-oxo-4-thia-1- azabicyclo[3.2.0]heptane-2-carboxylic acid | |
| Identifiers | |
| CAS number | |
| ATC code | J01 Template:ATCvet |
| PubChem | |
| DrugBank | |
| Chemical data | |
| Formula | Template:OrganicBox atomTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox |
| Mol. mass | 401.436 g/mol |
| SMILES | & |
| Physical data | |
| Density | 1.49 g/cm³ |
| Boiling point | 686.8 °C (1268 °F) |
| Pharmacokinetic data | |
| Bioavailability | ? |
| Metabolism | ? |
| Half life | ? |
| Excretion | ? |
| Therapeutic considerations | |
| Pregnancy cat. |
? |
| Legal status | |
| Routes | ? |
Mechanism of Action
- Penicillinase-resistant penicillins exert a bactericidal action against penicillin susceptible microorganisms during the state of active multiplication. All penicillins inhibit the biosynthesis of the bacterial cell wall.
Structure
- Oxacillin Injection, USP is a sterile injectable product containing oxacillin which is added as oxacillin sodium, a semisynthetic penicillin derived from the penicillin nucleus, 6-aminopenicillanic acid.
- The structural formula of oxacillin sodium is as follows:
- Oxacillin Injection, USP is a frozen, iso-osmotic, sterile, nonpyrogenic premixed 50 mL solution containing 1 g or 2 g of oxacillin added as oxacillin sodium. Dextrose, USP has been added to the above dosages to adjust osmolality (approximately 1.5 g and 300 mg as dextrose hydrous to the 1 g and 2 g dosages respectively).
- Sodium Citrate Hydrous, USP has been added as a buffer (approximately 150 mg and 300 mg to the 1 g and 2 g dosages, respectively). The pH has been adjusted with hydrochloric acid and may have been adjusted with sodium hydroxide. The pH is 6.5 (6.0 to 8.5). The solution is intended for intravenous use after thawing to room temperature.
- This GALAXY container (PL 2040) is fabricated from a specially designed multilayer plastic (PL 2040). Solutions are in contact with the polyethylene layer of this container and can leach out certain chemical components of the plastic in very small amounts within the expiration period. The suitability of the plastic has been confirmed in tests in animals according to the USP biological tests for plastic containers, as well as by tissue culture toxicity studies.
Pharmacodynamics
There is limited information regarding Pharmacodynamics of Oxacillin in the drug label.
Pharmacokinetics
There is limited information regarding Pharmacokinetics of Oxacillin in the drug label.
Nonclinical Toxicology
There is limited information regarding Nonclinical Toxicology of Oxacillin in the drug label.
Clinical Studies
There is limited information regarding Oxacillin Clinical Studies in the drug label.
How Supplied
- Oxacillin Injection, USP is supplied as a premixed frozen iso-osmotic solution in 50 mL single dose GALAXY plastic containers as follows:
- 2G3538 NDC 0338-1013-41 1 gram oxacillin
- 2G3539 NDC 0338-1015-41 2 grams oxacillin
Storage
- Store at or below -20°C/-4°F
Images
Drug Images
{{#ask: Page Name::Oxacillin |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
{{#ask: Label Page::Oxacillin |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
There is limited information regarding Patient Counseling Information of Oxacillin in the drug label.
Precautions with Alcohol
- Alcohol-Oxacillin interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
- OXACILLIN®[1]
Look-Alike Drug Names
There is limited information regarding Oxacillin Look-Alike Drug Names in the drug label.
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
{{#subobject:
|Label Page=Oxacillin |Label Name=Ox02.jpg
}}
{{#subobject:
|Label Page=Oxacillin |Label Name=Ox03.jpg
}}
{{#subobject:
|Label Page=Oxacillin |Label Name=DailyMed - OXACILLIN- oxacillin sodium injection, solution .png
}}