Nebivolol
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Sheng Shi, M.D. [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Nebivolol is a Beta-Adrenergic Blocker that is FDA approved for the {{{indicationType}}} of Hypertension. Common adverse reactions include Nausea, Dizziness, Headache, Somnolence.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Hypertension
- Dosing information
- The dose of BYSTOLIC must be individualized to the needs of the patient.
- Recommended starting dosage: 5 mg PO qd, with or without food, as monotherapy or in combination with other agents.
- For patients requiring further reduction in blood pressure, the dose can be increased at 2-week intervals up to 40 mg. A more frequent dosing regimen is unlikely to be beneficial.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
Patients with a history of MI or ACS and reduced ejection fraction (EF)
- Class of Recommendation: Class I
- Level of Evidence: Level B
- Dosing Information
- Not applicable
Non–Guideline-Supported Use
Congestive heart failure
- Dosing information
- 1.25 mg PO qd 15642700
- 10 mg PO qd 17584562
Prophylaxis of Migraine
- Dosing information
- 5 mg/day 18184294
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
FDA Package Insert for Nebivolol contains no information regarding FDA-labeled indications and dosage information for children.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Nebivolol sandbox in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Nebivolol sandbox in pediatric patients.
Contraindications
BYSTOLIC is contraindicated in the following conditions:
- Severe bradycardia
- Heart block greater than first degree
- Patients with cardiogenic shock
- Decompensated cardiac failure
- Sick sinus syndrome (unless a permanent pacemaker is in place)
- Patients with severe hepatic impairment (Child-Pugh >B)
- Patients who are hypersensitive to any component of this product.
Warnings
Abrupt Cessation of Therapy
Do not abruptly discontinue BYSTOLIC therapy in patients with coronary artery disease. Severe exacerbation of angina, myocardial infarction and ventricular arrhythmias have been reported in patients with coronary artery disease following the abrupt discontinuation of therapy with β-blockers. myocardial infarction and ventricular arrhythmias may occur with or without preceding exacerbation of the angina pectoris. Caution patients without overt coronary artery disease against interruption or abrupt discontinuation of therapy. As with other β-blockers, when discontinuation of BYSTOLIC is planned, carefully observe and advise patients to minimize physical activity. Taper BYSTOLIC over 1 to 2 weeks when possible. If the angina worsens or acute coronary insufficiency develops, re-start BYSTOLIC promptly, at least temporarily.
angina and Acute myocardial infarction
BYSTOLIC was not studied in patients with angina pectoris or who had a recent MI.
bronchospastic Diseases
In general, patients with bronchospastic diseases should not receive β-blockers.
Anesthesia and Major Surgery
Because beta-blocker withdrawal has been associated with an increased risk of mi and chest pain, patients already on beta-blockers should generally continue treatment throughout the perioperative period. If BYSTOLIC is to be continued perioperatively, monitor patients closely when anesthetic agents which depress myocardial function, such as ether, cyclopropane, and trichloroethylene, are used. If β-blocking therapy is withdrawn prior to major surgery, the impaired ability of the heart to respond to reflex adrenergic stimuli may augment the risks of general anesthesia and surgical procedures.
The β-blocking effects of BYSTOLIC can be reversed by β-agonists, e.g., dobutamine or isoproterenol. However, such patients may be subject to protracted severe hypotension. Additionally, difficulty in restarting and maintaining the heartbeat has been reported with β-blockers.
Diabetes and hypoglycemia
β-blockers may mask some of the manifestations of hypoglycemia, particularly tachycardia. Nonselective β-blockers may potentiate insulin-induced hypoglycemia and delay recovery of serum glucose levels. It is not known whether nebivolol has these effects. Advise patients subject to spontaneous hypoglycemia and diabetic patients receiving insulin or oral hypoglycemic agents about these possibilities.
Thyrotoxicosis
β-blockers may mask clinical signs of hyperthyroidism, such as tachycardia. Abrupt withdrawal of β-blockers may be followed by an exacerbation of the symptoms of hyperthyroidism or may precipitate a thyroid storm.
Peripheral Vascular Disease
β-blockers can precipitate or aggravate symptoms of arterial insufficiency in patients with peripheral vascular disease.
Non-dihydropyridine Calcium Channel Blockers
Because of significant negative inotropic and chronotropic effects in patients treated with β-blockers and calcium channel blockers of the verapamiland diltiazem type, monitor the ECG and blood pressure in patients treated concomitantly with these agents.
Use with CYP2D6 Inhibitors
Nebivolol exposure increases with inhibition of CYP2D6 [see Drug Interactions (7)]. The dose of BYSTOLIC may need to be reduced.
Impaired Renal Function
Renal clearance of nebivolol is decreased in patients with severe renal impairment. BYSTOLIC has not been studied in patients receiving dialysis [see Clinical Pharmacology (12.4) and Dosage and Administration (2.1)].
Impaired Hepatic Function
Metabolism of nebivolol is decreased in patients with moderate hepatic impairment. BYSTOLIC has not been studied in patients with severe hepatic impairment [see Clinical Pharmacology (12.4) and Dosage and Administration (2.1)].
Risk of Anaphylactic Reactions
While taking β-blockers, patients with a history of severe anaphylactic reactions to a variety of allergens may be more reactive to repeated accidental, diagnostic, or therapeutic challenge. Such patients may be unresponsive to the usual doses of epinephrine used to treat allergic reactions.
Pheochromocytoma
In patients with known or suspected pheochromocytoma, initiate an α-blocker prior to the use of any β-blocker.
Adverse Reactions
Clinical Trials Experience
BYSTOLIC has been evaluated for safety in patients with hypertensionand in patients with heart failure. The observed adverse reaction profile was consistent with the pharmacology of the drug and the health status of the patients in the clinical trials. Adverse reactions reported for each of these patient populations are provided below. Excluded are adverse reactions considered too general to be informative and those not reasonably associated with the use of the drug because they were associated with the condition being treated or are very common in the treated population.
The data described below reflect worldwide clinical trial exposure to BYSTOLIC in 6545 patients, including 5038 patients treated for hypertensionand the remaining 1507 subjects treated for other cardiovascular diseases. Doses ranged from 0.5 mg to 40 mg. Patients received BYSTOLIC for up to 24 months, with over 1900 patients treated for at least 6 months, and approximately 1300 patients for more than one year.
HYPERTENSION: In placebo-controlled clinical trials comparing BYSTOLIC with placebo, discontinuation of therapy due to adverse reactions was reported in 2.8% of patients treated with nebivolol and 2.2% of patients given placebo. The most common adverse reactions that led to discontinuation of BYSTOLIC were headache (0.4%), nausea (0.2%) and bradycardia (0.2%).
Table 1 lists treatment-emergent adverse reactions that were reported in three 12-week, placebo-controlled monotherapy trials involving 1597 hypertensive patients treated with either 5 mg, 10 mg, or 20-40 mg of BYSTOLIC and 205 patients given placebo and for which the rate of occurrence was at least 1% of patients treated with nebivolol and greater than the rate for those treated with placebo in at least one dose group.
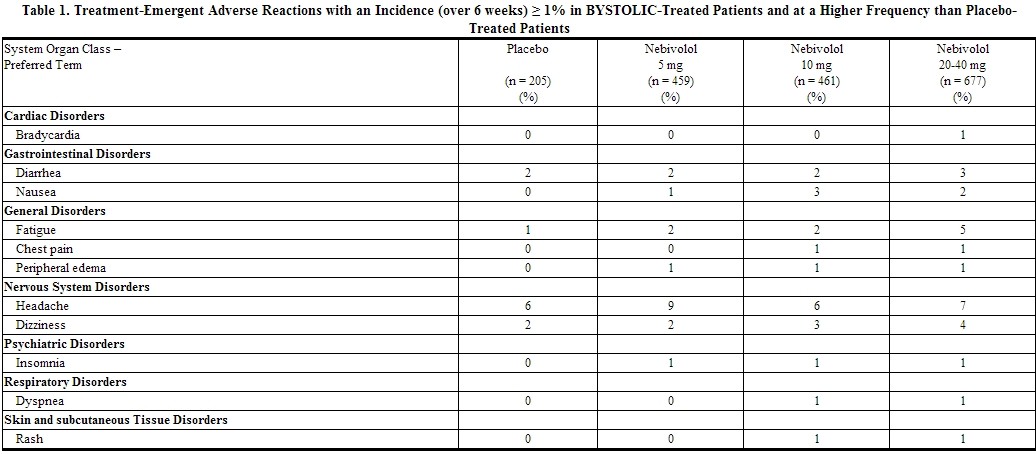
Listed below are other reported adverse reactions with an incidence of at least 1% in the more than 4300 patients treated with BYSTOLIC in controlled or open-label trials except for those already appearing in Table 1, terms too general to be informative, minor symptoms, or adverse reactions unlikely to be attributable to drug because they are common in the population. These adverse reactions were in most cases observed at a similar frequency in placebo-treated patients in the controlled studies.
Body as a Whole: asthenia.
Gastrointestinal System Disorders: abdominal pain
Metabolic and Nutritional Disorders: hypercholesterolemia
Nervous System Disorders: paraesthesia
Laboratory Abnormalities
In controlled monotherapy trials of hypertensive patients, BYSTOLIC was associated with an increase in BUN, uric acid, triglycerides and a decrease in HDL cholesterol and platelet count.
Postmarketing Experience
The following adverse reactions have been identified from spontaneous reports of BYSTOLIC received worldwide and have not been listed elsewhere. These adverse reactions have been chosen for inclusion due to a combination of seriousness, frequency of reporting or potential causal connection to BYSTOLIC. Adverse reactions common in the population have generally been omitted. Because these adverse reactions were reported voluntarily from a population of uncertain size, it is not possible to estimate their frequency or establish a causal relationship to BYSTOLIC exposure: abnormal hepatic function (including increased AST, ALT and bilirubin), acute pulmonary edema, acute renal failure, atrioventricular block (both second and third degree), bronchospasm, erectile dysfunction, hypersensitivity (including urticaria, allergic vasculitis and rare reports of angioedema), myocardial infarction, pruritus, psoriasis, Raynaud's phenomenon, peripheral ischemia/claudication, somnolence, syncope, thrombocytopenia, various rashes and skin disorders, vertigo, and vomiting.
Drug Interactions
CYP2D6 Inhibitors
Use caution when BYSTOLIC is co-administered with CYP2D6 inhibitors (quinidine, propafenone, fluoxetine, paroxetine, etc.) [see Clinical Pharmacology (12.5)].
Hypotensive Agents
Do not use BYSTOLIC with other β-blockers. Closely monitor patients receiving catecholamine-depleting drugs, such as reserpine or guanethidine, because the added β-blocking action of BYSTOLIC may produce excessive reduction of sympathetic activity. In patients who are receiving BYSTOLIC and clonidine, discontinue BYSTOLIC for several days before the gradual tapering of clonidine.
Digitalis Glycosides
Both digitalis glycosides and β-blockers slow atrioventricular conduction and decrease heart rate. Concomitant use can increase the risk of bradycardia.
Calcium Channel Blockers
BYSTOLIC can exacerbate the effects of myocardial depressants or inhibitors of AV conduction, such as certain calcium antagonists (particularly of the phenylalkylamine [ verapamil ] and benzothiazepine [diltiazem] classes), or antiarrhythmic agents, such as disopyramide.
Use in Specific Populations
Pregnancy
Pregnancy Category (FDA): C Decreased pup body weights occurred at 1.25 and 2.5 mg/kg in rats, when exposed during the perinatal period (late gestation, parturition and lactation). At 5 mg/kg and higher doses (1.2 times the MRHD), prolonged gestation, dystocia and reduced maternal care were produced with corresponding increases in late fetal deaths and stillbirths and decreased birth weight, live litter size and pup survival. Insufficient numbers of pups survived at 5 mg/kg to evaluate the offspring for reproductive performance.
In studies in which pregnant rats were given nebivolol during organogenesis, reduced fetal body weights were observed at maternally toxic doses of 20 and 40 mg/kg/day (5 and 10 times the MRHD), and small reversible delays in sternal and thoracic ossification associated with the reduced fetal body weights and a small increase in resorption occurred at 40 mg/kg/day (10 times the MRHD). No adverse effects on embryo-fetal viability, sex, weight or morphology were observed in studies in which nebivolol was given to pregnant rabbits at doses as high as 20 mg/kg/day (10 times the MRHD).
Pregnancy Category (AUS):
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Nebivolol in women who are pregnant.
Labor and Delivery
Nebivolol caused prolonged gestation and dystocia at doses ≥ 5 mg/kg in rats (1.2 times the MRHD). These effects were associated with increased fetal deaths and stillborn pups, and decreased birth weight, live litter size and pup survival rate, events that occurred only when nebivolol was given during the perinatal period (late gestation, parturition and lactation).
No studies of nebivolol were conducted in pregnant women. Use BYSTOLIC during pregnancy only if the potential benefit justifies the potential risk to the fetus.
Nursing Mothers
Studies in rats have shown that nebivolol or its metabolites cross the placental barrier and are excreted in breast milk. It is not known whether this drug is excreted in human milk.
Because of the potential for β-blockers to produce serious adverse reactions in nursing infants, especially bradycardia, BYSTOLIC is not recommended during nursing.
Pediatric Use
Safety and effectiveness in pediatric patients have not been established. Pediatric studies in ages newborn to 18 years old have not been conducted because of incomplete characterization of developmental toxicity and possible adverse effects on long-term fertility [see Nonclinical Toxicology (13.1)].
Geriatic Use
Of the 2800 patients in the U.S. sponsored placebo-controlled clinical hypertension studies, 478 patients were 65 years of age or older. No overall differences in efficacy or in the incidence of adverse events were observed between older and younger patients.
Gender
There is no FDA guidance on the use of Nebivolol with respect to specific gender populations.
Race
There is no FDA guidance on the use of Nebivolol with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Nebivolol in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Nebivolol in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Nebivolol in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Nebivolol in patients who are immunocompromised.
Heart Failure
In a placebo-controlled trial of 2128 patients (1067 BYSTOLIC, 1061 placebo) over 70 years of age with chronic heart failure receiving a maximum dose of 10 mg per day for a median of 20 months, no worsening of heart failure was reported with nebivolol compared to placebo. However, if heart failure worsens consider discontinuation of BYSTOLIC.
Administration and Monitoring
Administration
Oral
Monitoring
FDA Package Insert for Nebivolol contains no information regarding drug monitoring .
IV Compatibility
There is limited information regarding the compatibility of Nebivolol and IV administrations.
Overdosage
There is limited information regarding Nebivolol overdosage. If you suspect drug poisoning or overdose, please contact the National Poison Help hotline (1-800-222-1222) immediately.
Pharmacology
There is limited information regarding Nebivolol Pharmacology in the drug label.
Mechanism of Action
There is limited information regarding Nebivolol Mechanism of Action in the drug label.
Structure
There is limited information regarding Nebivolol Structure in the drug label.
Pharmacodynamics
There is limited information regarding Nebivolol Pharmacodynamics in the drug label.
Pharmacokinetics
There is limited information regarding Nebivolol Pharmacokinetics in the drug label.
Nonclinical Toxicology
There is limited information regarding Nebivolol Nonclinical Toxicology in the drug label.
Clinical Studies
There is limited information regarding Nebivolol Clinical Studies in the drug label.
How Supplied
There is limited information regarding Nebivolol How Supplied in the drug label.
Storage
There is limited information regarding Nebivolol Storage in the drug label.
Images
Drug Images
{{#ask: Page Name::Nebivolol |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
{{#ask: Label Page::Nebivolol |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
There is limited information regarding Nebivolol Patient Counseling Information in the drug label.
Precautions with Alcohol
Alcohol-Nebivolol sandbox interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
There is limited information regarding Nebivolol Brand Names in the drug label.
Look-Alike Drug Names
There is limited information regarding Nebivolol Look-Alike Drug Names in the drug label.
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.