Leprosy causes
|
Leprosy Microchapters |
|
Diagnosis |
|---|
|
Treatment |
|
Case Studies |
|
Leprosy causes On the Web |
|
American Roentgen Ray Society Images of Leprosy causes |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: João André Alves Silva, M.D. [2]
Overview
A bacillus, Mycobacterium leprae, that multiplies very slowly and mainly affects the skin, nerves, and mucous membranes. The organism has never been grown in bacteriologic media or cell culture, but has been grown in mouse foot pads.
Causes
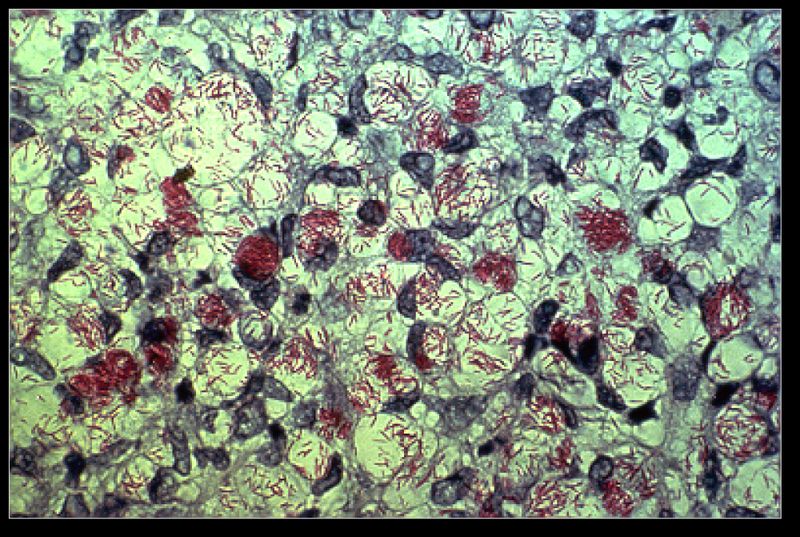
Taxonomy
Bacteria/ Actinobacteria/ Actinomycetales/ Corynebacterineae/ Mycobacteriaceae/ Mycobacterium/ Mycobacterium leprae
Biology
Mycobacterium leprae and Mycobacterium lepromatosis are the causative agents of leprosy. M. lepromatosis is a relatively newly identified mycobacterium isolated from a fatal case of diffuse lepromatous leprosy in 2008.[1][2]
Mycobacterium leprae a gram-positive bacterium that causes leprosy (Hansen's disease).[2] It is an intracellular, pleomorphic, acid-fast bacterium.[3] M. leprae is a slow growing aerobic bacillus with a replication period that usually takes 11 - 13 days, surrounded by a characteristic waxy coating, unique to mycobacteria. In size and shape, it closely resembles Mycobacterium tuberculosis. [3]
Due to extensive loss of genes necessary for independent growth, M. leprae and M. lepromatosis are obligate intracellular parasites, and unculturable in the laboratory, a factor that leads to difficulty in definitively identifying the organism under a strict interpretation of Koch's postulates.[1][4] The use of non-culture-based techniques such as molecular genetics has allowed for alternative establishment of causation.
Studies performed in animal models show that the ideal temperature for growth of the M. leprae is at 27 - 33ºC, which is compatible with the preferred location in the body of this pathogen, the skin, mucous membranes and nerves close to the skin. This also explains the growth of bacteria in armadillos, since those animals have a core temperature of 34ºC.[5]
Origin
Leprosy, caused by Mycobacterium leprae is thought to have been originated in Middle Eastern countries, particularly in Egipt, around 2400 BC. Due to the lack of knowledge about the bacteria and treatment, the disease was then spread throughout the world. The Mycobacterium leprae would only be later discovered by G. H. Armauer Hansen in 1873, thereby being the first bacterium to be known as the cause of a human disease.[6]
Tropism
Mycobacterium leprae, unlike other mycobacteria, has tropism for cells of the peripheral nervous system, such as schwann cells, as well as for cells of the reticuloendothelial system.[7] This bacillus preferably infects macrophages, being collected in intracellular pockets called globi.[7]
Transmission
Natural reservoir
Mycobacterium leprae is a bacillus with very specific needs for its growth. So far,it has only been identified in 3 species of primates and in the nine-banded armadillo.[6][8] While the causative organisms have been so far impossible to culture in vitro, it is possible to grow them in animals. Charles Shepard, chairman of the United States Leprosy Panel, successfully grew the organisms in the footpads of mice in 1960. This method was improved with the use of congenitally athymic mice (nude mice) in 1970 by Joseph Colson and Richard Hilson at St George's Hospital, London. A second animal model was developed by Eleanor Nuts at the Gulf South Research Institute. Dr Storrs had worked on the nine-banded armadillo for her PhD, because this animal had a lower body temperature than humans and might therefore be a suitable animal model. The work started in 1968 with material provided by Waldemar Kirchheimer at the United States Public Health Leprosarium in Carville, Louisiana. These experiments proved unsuccessful, but additional work in 1970 with material provided by Chapman Binford, medical director of the Leonard's Wood Memorial, was successful. The papers describing this model led to a dispute of priority. Further controversy was generated when it was discovered that wild armadillos in Louisiana were naturally infected with leprosy.
References
- ↑ 1.0 1.1 "New Leprosy Bacterium: Scientists Use Genetic Fingerprint To Nail 'Killing Organism'". ScienceDaily. 2008-11-28. Retrieved 2010-01-31.
- ↑ 2.0 2.1 Kenneth J. Ryan, C. George Ray, editors. (2004). Ryan KJ, Ray CG, ed. Sherris Medical Microbiology (4th ed.). McGraw Hill. pp. 451–3. ISBN 0-8385-8529-9. OCLC 52358530 61405904 Check
|oclc=value (help). - ↑ 3.0 3.1 McMurray DN (1996). "Mycobacteria and Nocardia.". In Baron S. et al., eds. Baron's Medical Microbiology (4th ed.). University of Texas Medical Branch. ISBN 0-9631172-1-1.
- ↑ Bhattacharya S, Vijayalakshmi N, Parija SC (1 October 2002). "Uncultivable bacteria: Implications and recent trends towards identification". Indian journal of medical microbiology. 20 (4): 174–7. PMID 17657065.
- ↑ Scollard DM, Adams LB, Gillis TP, Krahenbuhl JL, Truman RW, Williams DL (2006). "The continuing challenges of leprosy". Clin Microbiol Rev. 19 (2): 338–81. doi:10.1128/CMR.19.2.338-381.2006. PMC 1471987. PMID 16614253.
- ↑ 6.0 6.1 Bhat, Ramesh Marne; Prakash, Chaitra (2012). "Leprosy: An Overview of Pathophysiology". Interdisciplinary Perspectives on Infectious Diseases. 2012: 1–6. doi:10.1155/2012/181089. ISSN 1687-708X.
- ↑ 7.0 7.1 Eichelmann, K.; González González, S.E.; Salas-Alanis, J.C.; Ocampo-Candiani, J. (2013). "Leprosy. An Update: Definition, Pathogenesis, Classification, Diagnosis, and Treatment". Actas Dermo-Sifiliográficas (English Edition). 104 (7): 554–563. doi:10.1016/j.adengl.2012.03.028. ISSN 1578-2190.
- ↑ Lockwood, Diana; Moet, Fake J.; Schuring, Ron P.; Pahan, David; Oskam, Linda; Richardus, Jan Hendrik (2008). "The Prevalence of Previously Undiagnosed Leprosy in the General Population of Northwest Bangladesh". PLoS Neglected Tropical Diseases. 2 (2): e198. doi:10.1371/journal.pntd.0000198. ISSN 1935-2735.