High density lipoprotein biochemistry
|
High Density Lipoprotein Microchapters |
|
Diagnosis |
|---|
|
Treatment |
|
Clinical Trials |
|
Case Studies |
|
High density lipoprotein biochemistry On the Web |
|
American Roentgen Ray Society Images of High density lipoprotein biochemistry |
|
Risk calculators and risk factors for High density lipoprotein biochemistry |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Aarti Narayan, M.B.B.S [2]; Raviteja Guddeti, M.B.B.S. [3]
Biochemistry
Structure
| Lipoprotein | Density | Size | % Protein | Cholesterol in Plasma | Triglyceride in Fasting Plasma | Major Apolipoprotein |
| HDL | 1.063 - 1.210 g/mL | 6 - 10 mm | 40 - 55% | 0.9 - 1.6 mmol/L | 0.1 - 0.2 mmol/L | apoA-I, apoA-II |
For more information about the biochemistry of all lipoproteins, click here.
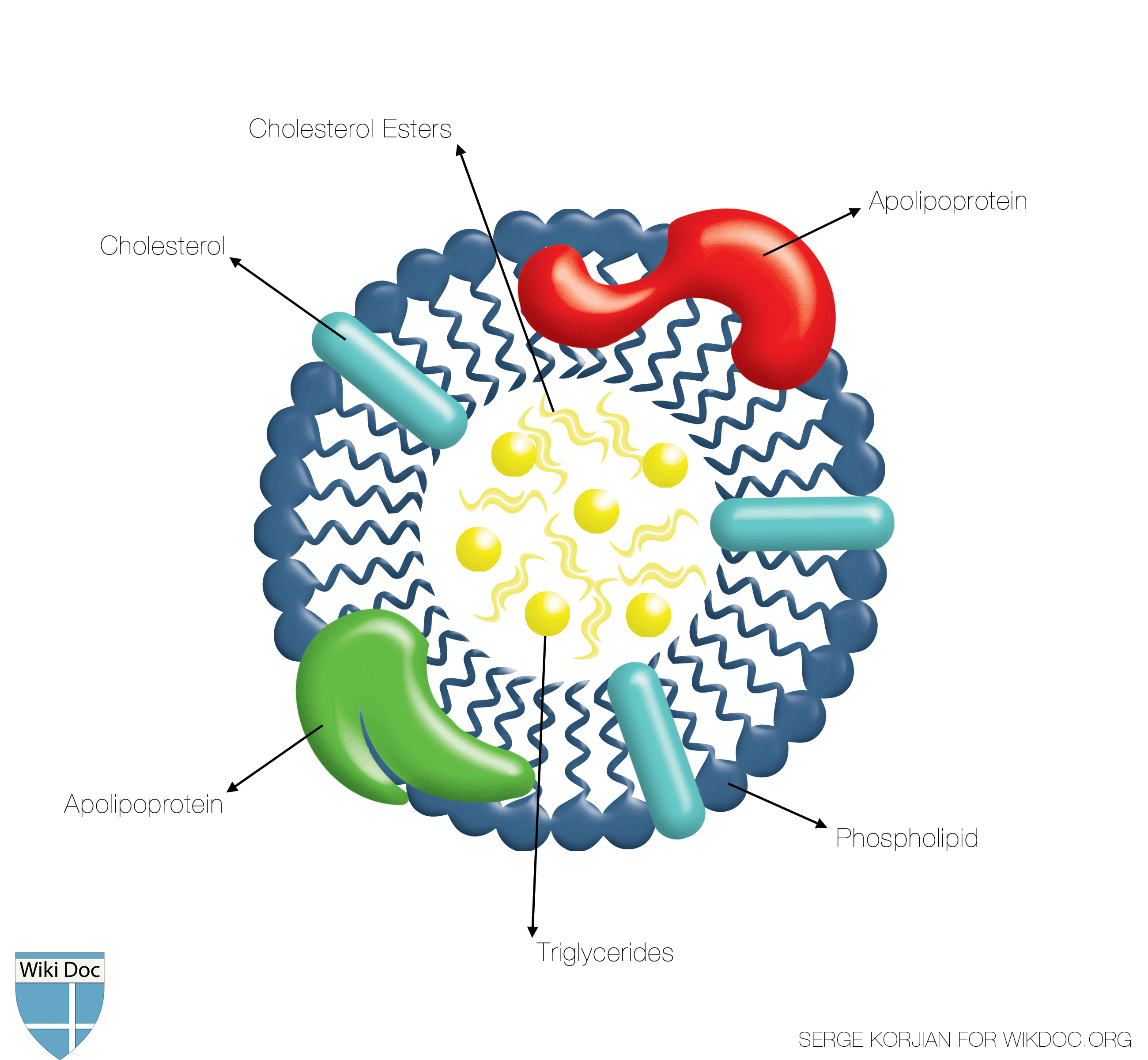
Shown below is a a schematic image depicting the structure of the HDL. Note that the inner core is made of triglyceride and cholesterol esters whereas the surface is made of amphiphilic phospholipids along with apolipoproteins.
- HDL are the smallest of the lipoproteins. They are the densest because they contain the highest proportion of protein. They contain the A class of apolipoproteins. Apolipoprotein A-I is the main protein of HDL that remove excess cell cholesterol and protect against atherosclerosis.[1]
- The liver synthesizes these lipoproteins as complexes of apolipoproteins and phospholipids, which resemble cholesterol-free flattened spherical lipoprotein particles. They are capable of picking up cholesterol from cells they interact with.
- A plasma enzyme called lecithin-cholesterol acyltransferase (LCAT) converts the free cholesterol into cholesteryl ester (a more hydrophobic form of cholesterol) which is then sequestered into the core of the lipoprotein particle eventually making the newly synthesized HDL spherical. They increase in size as they circulate through the bloodstream and incorporate more cholesterol molecules into their structure.
- Thus it is the concentration of large HDL particles which more accurately reflects protective action, as opposed to the concentration of total HDL particles.[2] This ratio of large HDL to total HDL particles varies widely and is only measured by more sophisticated lipoprotein assays using either electrophoresis, originally developed in the 1970s, or newer Nuclear magnetic resonance (NMR) spectroscopy which was developed in the 1990s.
- HDL particles are not inherently protective. It is only the HDL particles which become the largest (actually picking up and carrying cholesterol) which are protective. There is no reliable relationship between total HDL and large HDL, and more sophisticated analyses which actually measure large HDL, not just total, correlate much better with clinical outcomes.[3]
- Many studies have postulated an association between cholesterol efflux from peripheral tissue, and Apo A-I HDL particles, whereas the HDL3 containing both Apo AI and A-II are less effective. [4] [5] [6]
HDL Receptors
- ABCA1 transporter: it is expressed in the peripheral tissues, intestine, liver and macrophage.[7] An increase in intracellular cholesterol content upregulates ABCA1 trnasporter which is responsible for cholesterol efflux from the intracellular pool.[8]
Enzymes Associated with HDL
Cholesterol Ester Transfer Protein (CETP)
- This protein mediates exchange of cholesterol between HDL particles, and triglyceride rich LDL and VLDL in both directions.
- It is normally present in both periphery and liver and functions to channel cholesterol to the liver for uptake and metabolism.
References
- ↑ Mahler, DA.; Shuhart, CR.; Brew, E.; Stukel, TA. (1991). "Ventilatory responses and entrainment of breathing during rowing". Med Sci Sports Exerc. 23 (2): 186–92. PMID 2017014. Unknown parameter
|month=ignored (help) - ↑ Kwiterovich PO. The Metabolic Pathways of High-Density Lipoprotein, Low-Density Lipoprotein, and Triglycerides: A Current Review. Am J Cardiol 2000;86(suppl):5L.
- ↑ Tran-Dinh A, Diallo D, Delbosc S; et al. (2013). "HDL and endothelial protection". British Journal of Pharmacology. doi:10.1111/bph.12174. PMID 23488589. Unknown parameter
|month=ignored (help) - ↑ Yin K, Tang SL, Yu XH; et al. (2013). "Apolipoprotein A-I inhibits LPS-induced atherosclerosis in ApoE-/- mice possibly via activated STAT3-mediated upregulation of tristetraprolin". Acta Pharmacologica Sinica. doi:10.1038/aps.2013.10. PMID 23564081. Unknown parameter
|month=ignored (help) - ↑ Mazer NA, Giulianini F, Paynter NP, Jordan P, Mora S (2013). "A Comparison of the Theoretical Relationship between HDL Size and the Ratio of HDL Cholesterol to Apolipoprotein A-I with Experimental Results from the Women's Health Study". Clinical Chemistry. doi:10.1373/clinchem.2012.196949. PMID 23426429. Unknown parameter
|month=ignored (help) - ↑ Kappelle PJ, Gansevoort RT, Hillege HJ, Wolffenbuttel BH, Dullaart RP (2013). "Common variation in cholesteryl ester transfer protein: relationship of first major adverse cardiovascular events with the apolipoprotein B/apolipoprotein A-I ratio and the total cholesterol/high-density lipoprotein cholesterol ratio". Journal of Clinical Lipidology. 7 (1): 56–64. doi:10.1016/j.jacl.2012.05.003. PMID 23351584. Unknown parameter
|month=ignored (help) - ↑ Fitzgerald, ML.; Mujawar, Z.; Tamehiro, N. (2010). "ABC transporters, atherosclerosis and inflammation". Atherosclerosis. 211 (2): 361–70. doi:10.1016/j.atherosclerosis.2010.01.011. PMID 20138281. Unknown parameter
|month=ignored (help) - ↑ Schwartz, K.; Lawn, RM.; Wade, DP. (2000). "ABC1 gene expression and ApoA-I-mediated cholesterol efflux are regulated by LXR". Biochem Biophys Res Commun. 274 (3): 794–802. doi:10.1006/bbrc.2000.3243. PMID 10924356. Unknown parameter
|month=ignored (help)