Hepatitis B pathophysiology: Difference between revisions
Sergekorjian (talk | contribs) |
Sergekorjian (talk | contribs) |
||
| Line 11: | Line 11: | ||
===Life Cycle=== | ===Life Cycle=== | ||
* The [[HBV]] [[virion]] binds to a receptor at the surface of the [[hepatocyte]].<ref name=WHO>{{cite web | title = Hepatitis B | url = http://www.who.int/csr/disease/hepatitis/HepatitisB_whocdscsrlyo2002_2.pdf }}</ref> | * The [[HBV]] [[virion]] binds to a receptor at the surface of the [[hepatocyte]].<ref name=WHO>{{cite web | title = Hepatitis B | url = http://www.who.int/csr/disease/hepatitis/HepatitisB_whocdscsrlyo2002_2.pdf }}</ref> | ||
| Line 24: | Line 22: | ||
* The new, mature, viral nucleocapsids can then follow two different [[intracellular]] pathways, one of which leads to the formation and secretion of new virions, whereas the other leads to amplification of the viral genome inside the cell nucleus. In the virion assembly pathway, the nucleocapsids reach the ER, where they associate with the envelope proteins and bud into the lumen of the ER, from which they are secreted via the Golgi apparatus out of the cell.<ref name=WHO>{{cite web | title = Hepatitis B | url = http://www.who.int/csr/disease/hepatitis/HepatitisB_whocdscsrlyo2002_2.pdf }}</ref> | * The new, mature, viral nucleocapsids can then follow two different [[intracellular]] pathways, one of which leads to the formation and secretion of new virions, whereas the other leads to amplification of the viral genome inside the cell nucleus. In the virion assembly pathway, the nucleocapsids reach the ER, where they associate with the envelope proteins and bud into the lumen of the ER, from which they are secreted via the Golgi apparatus out of the cell.<ref name=WHO>{{cite web | title = Hepatitis B | url = http://www.who.int/csr/disease/hepatitis/HepatitisB_whocdscsrlyo2002_2.pdf }}</ref> | ||
[[File:Hepatitis B life cycle.png|500px|thumb|center|Systematic Diagram of the Life Cycle of HBV <SMALL>Courtesy: ''[http://www.who.int/en/ World Health Organization]''<ref>{{Cite web | title = http://www.who.int/en/ | url = http://www.who.int/en/}}</ref></SMALL>]] | |||
===HBV and Antibody Nomenclature=== | ===HBV and Antibody Nomenclature=== | ||
Revision as of 19:04, 4 August 2014
|
Hepatitis B |
|
Diagnosis |
|
Treatment |
|
Case Studies |
|
Hepatitis B pathophysiology On the Web |
|
American Roentgen Ray Society Images of Hepatitis B pathophysiology |
|
Risk calculators and risk factors for Hepatitis B pathophysiology |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: João André Alves Silva, M.D. [2]
Overview
The intracellular hepatitis B virus is a non-cytopathic virus that causes little or no damage to the cell.[1] During HBV infection, the host immune response causes both hepatocellular damage and viral clearance. The HBV virion binds to a receptor at the surface of the hepatocyte and enters the cell, where it uses the host's cell mechanisms to replicate its genome and proteins.[1] Different viral antigens and antibodies are detected in serum throughout the course of the disease, such as: HBsAg, HBcAg, HBeAg, anti-HBs, anti-HBC and anti-HBe. Transmission occurs from exposure to infectious blood or body fluids. Hepatitis B is often associated with hepatocellular carcinoma. Immune complexes, such as surface antigen-antibody, are important in the pathogenesis of hepatitis B.
Pathogenesis
Immunopathogenesis
The host immune response is primarily responsible for both hepatocellular damage and viral clearance in patients with HBV infection.[1] While the innate immune response does not play a significant role in these processes, the adaptive immune response, particularly virus-specific cytotoxic T lymphocytes, contribute to nearly all of the liver injury associated with HBV infection.[1]
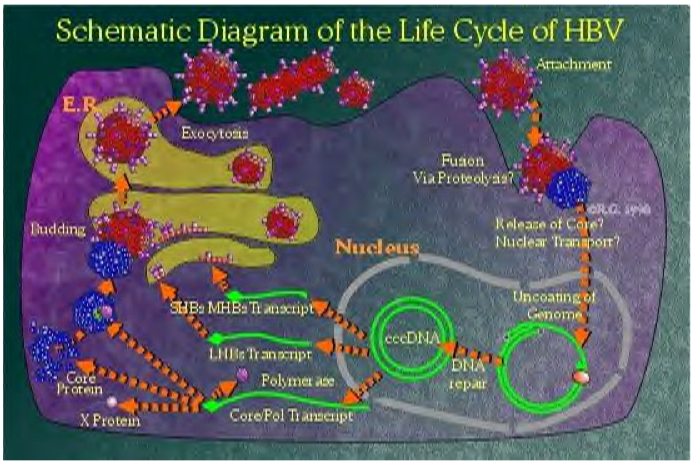
Life Cycle
- The HBV virion binds to a receptor at the surface of the hepatocyte.[1]
- Several cellular receptors have been identified, including the transferrin receptor, the asialoglycoprotein receptor molecule, and the human liver endonexin. However, the mechanism of HBsAg binding to a specific receptor to enter cells has not been established yet. Viral nucleocapsids enter the cell and reach the nucleus, where the viral genome is delivered.[1][2][3]
- In the nucleus, second-strand DNA synthesis is completed and the gaps in both strands are repaired to yield a covalently closed circular (ccc) supercoiled DNA molecule that serves as a template for transcription of four viral RNAs that are 3.5, 2.4, 2.1, and 0.7 kb long.[4][5]
- These transcripts are polyadenylated and transported to the cytoplasm, where they are translated into the viral nucleocapsid and precore antigen (C, pre-C), polymerase (P), envelope L (large), M (medium), S (small)), and transcriptional transactivating proteins (X).[6][7]
- The envelope proteins insert themselves as integral membrane proteins into the lipid membrane of the endoplasmic reticulum (ER). The 3.5 kb species, spanning the entire genome and termed pregenomic RNA (pgRNA), is packaged together with HBV polymerase and a protein kinase into core particles where it serves as a template for reverse transcription of negative-strand DNA. The RNA to DNA conversion takes place inside the particles.
- The new, mature, viral nucleocapsids can then follow two different intracellular pathways, one of which leads to the formation and secretion of new virions, whereas the other leads to amplification of the viral genome inside the cell nucleus. In the virion assembly pathway, the nucleocapsids reach the ER, where they associate with the envelope proteins and bud into the lumen of the ER, from which they are secreted via the Golgi apparatus out of the cell.[1]
HBV and Antibody Nomenclature
| Nomenclature | Full Name | Description |
|---|---|---|
| HBV | Hepatitis B Virus (complete infectious virion) | The 42 nm, double-shelled particle, that consists of a 7 nm thick outer shell and a 27 nm inner core. The core contains a small, circular, partially double-stranded DNA molecule and an endogenous DNA polymerase. This is the prototype agent for the family epadnaviridae.[1] |
| HBsAg | Hepatitis B Surface Antigen (envelope antigen) | The complex of antigenic determinants found on the surface of HBV and of 22 nm particles and tubular forms.[1] |
| HBcAg | Hepatitis B Core Antigen | The antigenic specificity.[1] |
| HBeAg | Hepatitis B e Antigen | The antigenic determinant that is closely associated with the nucleocapsid of HBV. It also circulates as a soluble protein in serum.[1] |
| Anti-HBs Anti-HBc Anti-HBe |
Antibody to HBsAg Antibody to HBcAg Antibody to HBeAg |
Specific antibodies that are produced in response to their respective antigenic determinants.[1] |
Transmission
- Transmission results from exposure to infectious blood or body fluids.[1]
- Possible forms of transmission include:[9]
- Unprotected sexual contact
- Blood transfusions
- Re-use of contaminated needles and syringes
- Vertical transmission from mother to child during childbirth (20% risk of transmission/ risk is as high as 90% if the mother is also positive for the hepatitis B e antigen)
- Transmission between family members within households, possibly by contact of nonintact skin or mucous membrane with secretions or saliva containing HBV.
Associated Conditions
Polyarthritis Nodosa
- One form of polyarthritis nodosa (PAN) is the hepatitis B virus-associated polyarthritis nodosa (HBV-PAN). Its occurence is attributed to the deposition of auto-immune complexes with excess antigen in tissues such as the kidneys, joints and GI tract.[10]
- Clinically it can be characterized as:[10]
- Renal involvement
- Renal vasculitis
- Rare relapses, never occurring after viral replication has stopped and seroconversion has occurred[10]
- GI tract involvement represents the greatest cause of death[10]
- Vaccination and blood safety have decreased the incidence of HBV-PAN[10]
Hepatocellular Carcinoma
- Hepatitis B is often associated with hepatocellular carcinoma.
- More than 85% of examined hepatocellular tumours harbor integrated HBV DNA, often multiple copies per cell. The viral DNA integrants are usually highly rearranged, with deletions, inversions, and sequence reiterations all commonly observed. Most of these rearrangements ablate viral gene expression, but the integrations alter the host DNA.[11][1][12]
- Every cell in the tumour contains an identical complement of HBV insertions. This implies that the integration event(s) preceded the clonal expansion of the cells.
- There is no similarity in the pattern of integration between different tumours, and variation is seen both in the integration site(s) and in the number of copies or partial copies of the viral genome.[1]
Coinfections
Hepatitis D
- Hepatitis Delta virus (HDV) is a defective virus that is only infectious in the presence of active HBV infection.[1]
- HDV infection occurs as either coinfection with HBV or superinfection of an HBV carrier:[1][13]
- Coinfection - usually resolves
- Superinfection - causes frequently chronic HDV infection and chronic active hepatitis.
- Both types of infections may cause fulminant hepatitis.[1]
- Routes of transmission are similar to those of HBV.[1]
- Preventing acute and chronic HBV infection of susceptible persons by vaccination will also prevent HDV infection.[1][14]
HIV
- About 10% of people living with HIV in the U.S. are coinfected with HBV.[15]
- People living with HIV who are coinfected with HBV are at increased risk for serious, life-threatening health complications. HIV/HBV coinfection can also complicate the management of HIV infection.[15]
- Hepatitis B is preventable with a vaccine and HBV vaccination is recommended for people who are at risk for or living with HIV who have tested negative for HBV
- Persons infected with HIV are more likely to develop persistent infection with HBV.[1]
- One of the factors that may reduce the immunogenicity of hepatitis vaccines, along with age (>40 years), gender, weight, genetics, haemodialysis, immunosuppression and tobacco smoking.[1]
Pathology
Immune complexes, such as surface antigen-antibody, are important in the pathogenesis of other disease syndromes characterized by severe damage of blood vessels:
- Acute necrotizing vasculitis (polyarteritis nodosa)[1]
With high fever, anemia, leucocytosis, arthralgia, arthritis, renal disease, hypertension, heart disease, gastrointestinal disease, skin manifestations, neurologic disorders. Highly variable disease with mortality rate of 40% within 3 years unless treated. The diagnosis is established by angiography.[1]
- Membranous glomerulonephritis:
Present in both adults and children. Remission of nephropathy occurs in 85 to 90% of cases over a period of 9 years and is associated with clearance of HBeAg from serum.[1]
- Papular acrodermatitis of childhood (Gianotti-Crosti syndrome)
Distinctive disease of childhood. Skin lesions, lentil-sized, flat, erythematous, and papular eruptions localized to the face and extremities, last 15 to 20 days. The disease is accompanied by generalized lymphadenopathy, hepatomegaly, and acute anicteric hepatitis B of ayw subtype.[1]
Why only a small proportion of patients with circulating complexes develop vasculitis or polyarteritis is still not clear.[1]
References
- ↑ 1.00 1.01 1.02 1.03 1.04 1.05 1.06 1.07 1.08 1.09 1.10 1.11 1.12 1.13 1.14 1.15 1.16 1.17 1.18 1.19 1.20 1.21 1.22 1.23 1.24 1.25 1.26 "Hepatitis B" (PDF).
- ↑ Nathanson, Neal (1997). Viral pathogenesis. Philadelphia: Lippincott-Raven. ISBN 0781702976.
- ↑ Guidotti LG, Martinez V, Loh YT, Rogler CE, Chisari FV (1994). "Hepatitis B virus nucleocapsid particles do not cross the hepatocyte nuclear membrane in transgenic mice". J Virol. 68 (9): 5469–75. PMC 236947. PMID 8057429.
- ↑ Nathanson, Neal (1997). Viral pathogenesis. Philadelphia: Lippincott-Raven. ISBN 0781702976.
- ↑ Plotkin, Stanley (1999). Vaccines. Philadelphia: W.B. Saunders Co. ISBN 0721674437.
- ↑ Nathanson, Neal (1997). Viral pathogenesis. Philadelphia: Lippincott-Raven. ISBN 0781702976.
- ↑ Mandell, Gerald (2005). Mandell, Douglas, and Bennett's principles and practice of infectious diseases. New York: Elsevier/Churchill Livingstone. ISBN 0443066434.
- ↑ "http://www.who.int/en/". External link in
|title=(help) - ↑ name="pmid791124">Petersen NJ, Barrett DH, Bond WW, Berquist KR, Favero MS, Bender TR, Maynard JE (1976). "Hepatitis B surface antigen in saliva, impetiginous lesions, and the environment in two remote Alaskan villages". Appl. Environ. Microbiol. 32 (4): 572–574. PMID 791124.
- ↑ 10.0 10.1 10.2 10.3 10.4 Guillevin L, Mahr A, Callard P, Godmer P, Pagnoux C, Leray E; et al. (2005). "Hepatitis B virus-associated polyarteritis nodosa: clinical characteristics, outcome, and impact of treatment in 115 patients". Medicine (Baltimore). 84 (5): 313–22. PMID 16148731.
- ↑ Mandell, Gerald (2005). Mandell, Douglas, and Bennett's principles and practice of infectious diseases. New York: Elsevier/Churchill Livingstone. ISBN 0443066434.
- ↑ Fields, Bernard (2007). Fields virology. Philadelphia: Wolters Kluwer Health/Lippincott Williams & Wilkins. ISBN 0781760607.
- ↑ Mandell, Gerald (2010). Mandell, Douglas, and Bennett's principles and practice of infectious diseases. Philadelphia, PA: Churchill Livingstone/Elsevier. ISBN 0443068399.
- ↑ Mandell, Gerald (2010). Mandell, Douglas, and Bennett's principles and practice of infectious diseases. Philadelphia, PA: Churchill Livingstone/Elsevier. ISBN 0443068399.
- ↑ 15.0 15.1 "Hepatitis".