Gingivitis: Difference between revisions
| Line 614: | Line 614: | ||
*Treatment approach: An interprofessional approach is required to identify the causes of gingivitis and to intervene at an early stage before its progression to periodontitis. | *Treatment approach: An interprofessional approach is required to identify the causes of gingivitis and to intervene at an early stage before its progression to periodontitis. | ||
*Aim: To restore the inflamed tissues to clinical health and then to maintain clinically healthy gingivae and subsequently preventing periodontitis. | *Aim: To restore the inflamed tissues to clinical health and then to maintain clinically healthy gingivae and subsequently preventing periodontitis. Periodontitis has also been linked to diabetes, arteriosclerosis, osteoporosis, pancreatic cancer and pre-term low birth weight babies. | ||
*Stepwise approach: | *Stepwise approach: | ||
**A dentist or dental hygienist will perform a thorough cleaning of the teeth and gums and remove localized factors initiating the inflammatory response. This includes scaling to thoroughly remove biofilm and deposits on the tooth structure and laser decontamination of the sulcus if possible. The removal of plaque is usually not painful, and the inflammation of the gums should be gone between one and two weeks. | **A dentist or dental hygienist will perform a thorough cleaning of the teeth and gums and remove localized factors initiating the inflammatory response. This includes scaling to thoroughly remove biofilm and deposits on the tooth structure and laser decontamination of the sulcus if possible. The removal of plaque is usually not painful, and the inflammation of the gums should be gone between one and two weeks. | ||
Revision as of 19:24, 10 September 2020
| Gingivitis | |
 | |
|---|---|
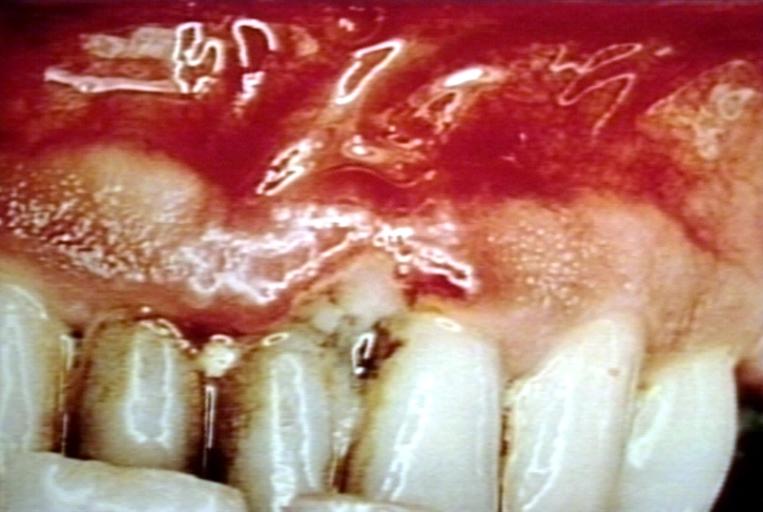
| Trench mouth. Necrotizing gingivitis Image courtesy of Professor Peter Anderson DVM PhD and published with permission. © PEIR, University of Alabama at Birmingham, Department of Pathology | |
| ICD-10 | K05.0-K05.1 |
|
WikiDoc Resources for Gingivitis |
|
Articles |
|---|
|
Most recent articles on Gingivitis |
|
Media |
|
Evidence Based Medicine |
|
Clinical Trials |
|
Ongoing Trials on Gingivitis at Clinical Trials.gov Clinical Trials on Gingivitis at Google
|
|
Guidelines / Policies / Govt |
|
US National Guidelines Clearinghouse on Gingivitis
|
|
Books |
|
News |
|
Commentary |
|
Definitions |
|
Patient Resources / Community |
|
Patient resources on Gingivitis Discussion groups on Gingivitis Patient Handouts on Gingivitis Directions to Hospitals Treating Gingivitis Risk calculators and risk factors for Gingivitis
|
|
Healthcare Provider Resources |
|
Causes & Risk Factors for Gingivitis |
|
Continuing Medical Education (CME) |
|
International |
|
|
|
Business |
|
Experimental / Informatics |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Ogheneochuko Ajari, MB.BS, MS [2] Jaspinder Kaur, MBBS[3]
Synonyms and keywords:
Overview
Gingivitis ("inflammation of the gums") (gingiva) around the teeth is a general term for gingival diseases affecting the gingiva (gums). As generally used, the term gingivitis refers to gingival inflammation induced by bacterial biofilms (also called plaque) adherent to tooth surfaces. It has been defined as the reversible dental plaque‑induced inflammation of the gingiva without detectable bone loss or clinical attachment loss. It is frequently encountered in dental practice, affected people of all ages and describes the condition of the dental soft tissue. It may also be associated with dental plaque only while gingival diseases modified by systemic factors associated with the endocrine system. The etiology of gingivitis is multi‑factorial and the result of more than one factor acting together. A wide range of factors has been identified as significantly associated with gingivitis including the presence of bacteria biofilm, genetic, socioeconomic, demographic, iatrogenic, and behavioral factors. These factors seem to influence the process, making it difficult to identify the risk factors. The most important factor that has been associated with gingivitis is plaque accumulation on the dental surface, resulting in an inflammatory reaction, with clinical signs of redness, edema, gingival bleeding and sometimes pain, whereas as the condition persists that were initially edematous may become more fibrotic. Gingivitis is generally regarded as a site-specific inflammatory condition initiated by dental biofilm accumulation and characterized by gingival redness and edema5 and the absence of periodontal attachment loss. Gingivitis is commonly painless, rarely leads to spontaneous bleeding, and is often characterized by subtle clinical changes, resulting in most patients being unaware of the disease or unable to recognize it. It is generally accepted that periodontal disease in children, adolescents, and adults begins as gingivitis, which progresses, only in some individuals, to periodontitis. Notwithstanding the reversibility of the gingivitis-elicited tissue changes, gingivitis holds particular clinical significance because it is considered the precursor of periodontitis, a disease characterized by gingival inflammation combined with connective tissue attachment and bone loss. However, it is a reversible disease. Therapy is aimed primarily at reduction of etiologic factors to reduce or eliminate inflam¬mation, thereby allowing gingival tissues to heal. Appropriate supportive periodontal maintenance that includes personal and professional care is important in preventing re-initiation of inflammation.
Classification
The 2017 World Workshop has classified the gingival diseases into two broad categories:
| Periodontal Health |
|---|
|
| Gingivitis—Dental Plaque-induced |
|
| Gingival Disease—Non-dental Plaque-induced |
|
Stages
The condition gingivitis undergoes through four different stages before progressing to periodontitis if not treated. The different stages of gingivitis were first explained by Page and Schroeder in 1976.
| Stage | Differentiating features |
|---|---|
| Initial: 24-48 hours |
|
| Early: 4-7 days |
|
| Established: 2-3 weeks |
|
| Advanced |
|
Pathophysiology
Gingivitis is usually caused by bacterial plaque that accumulates in the spaces between the gums and the teeth and in calculus (tartar) that forms on the teeth. These accumulations may be tiny, even microscopic, but the bacteria in them produce foreign chemicals and toxins that cause inflammation of the gums around the teeth. This inflammation can, over the years, cause deep pockets between the teeth and gums and loss of bone around teeth otherwise known as periodontitis.
Since the bone in the jaws holds the teeth into the jaws, the loss of bone can cause teeth over the years to become loose and eventually to fall out or need to be extracted because of acute infection. Regular cleanings (correctly termed periodontal debridement, scaling or root planing) below the gum line, best accomplished professionally by a dental hygienist or dentist, disrupt this plaque biofilm and remove plaque retentive calculus (tartar) to help prevent inflammation. Once cleaned, plaque will begin to grow on the teeth within hours. However, it takes approximately 3 months for the pathogenic type of bacteria (typically gram negative anaerobes and spirochetes) to grow back into the deep pockets and restart the inflammatory process. Calculus (tartar) may start to reform within 24 hours. Ideally, scientific studies show that all people with deep periodontal pockets (greater than 5mm) should have the pockets between their teeth and gums cleaned by a dental hygienist or dentist every 3-4 months.
People with a healthy periodontium (gums, bone and ligament) or people with gingivitis only require periodontal debridement every 6 months. However, many dental professionals only recommend periodontal debridement (cleanings) every 6 months, because this has been the standard advice for decades, and because the benefits of regular periodontal debridement (cleanings) are too subtle for many patients to notice without regular education from the dental hygienist or dentist. If the inflammation in the gums becomes especially well-developed, it can invade the gums and allow tiny amounts of bacteria and bacterial toxins to enter the bloodstream. The patient may not be able to notice this, but studies suggest this can result in a generalized increase in inflammation in the body cause possible long term heart problems. Periodontitis has also been linked to diabetes, arteriosclerosis, osteoporosis, pancreatic cancer and pre-term low birth weight babies.
When the teeth are not cleaned properly by regular brushing and flossing, bacterial plaque accumulates, and becomes mineralized by calcium and other minerals in the saliva transforming it into a hard material called calculus (tartar) which harbors bacteria and irritates the gingiva (gums). Also, as the bacterial plaque biofilm becomes thicker this creates an anoxygenic environment which allows more pathogenic bacteria to flourish and release toxins and cause gingival inflammation. Alternatively, excessive injury to the gums caused by very vigorous brushing may lead to recession, inflammation and infection. Pregnancy, uncontrolled diabetes mellitus and the onset of puberty increase the risk of gingivitis, due to hormonal changes that may increase the susceptibility of the gums or alter the composition of the dentogingival microflora. The risk of gingivitis is increased by misaligned teeth, the rough edges of fillings, and ill fitting or unclean dentures, bridges, and crowns. This is due to their plaque retentive properties. The drug phenytoin, birth control pills, and ingestion of heavy metals such as lead and bismuth may also cause gingivitis.
The sudden onset of gingivitis in a normal, healthy person should be considered an alert to the possibility of an underlying viral aetiology, although most systemically healthy individuals have gingivitis in some area of their mouth, usually due to inadequate brushing and flossing.
Inadequate oral hygiene leads to bacterial plaque accumulation which, if not removed, triggers an acute inflammatory response within less than a week. This is the initial stage of gingivitis characterized by an increase in gingival crevicular fluid and number of neutrophils. The collagen fibers start destroying along with the deposition of fibrin. At one week, gingivitis proceeds to an early stage with the transition from neutrophilic to lymphocytic infiltration predominantly. Further progression into the chronic stage leads to an established lesion with predominantly plasma cells and B lymphocytes. As it progresses, pocket formation occurs, resulting in separation of gingiva from the tooth. The persistent inflammation leads to the breakdown of the periodontal ligament and resorption of the adjacent alveolar bone, which may ultimately result in tooth loss.
Causes
Life Threatening Causes
Life-threatening causes include conditions which may result in death or permanent disability within 24 hours if left untreated.
Common Causes
Causes by Organ System
Causes in Alphabetical Order
| A | B | C | D | E |
|---|---|---|---|---|
| F | G | H | I | K |
| L | M | N | O | P |
| R | S | T | V | Z |
Differentiating Gingivitis from other Diseases
| Differentiating condition | Differentiating sign and symptoms | Differentiating features |
|---|---|---|
| Oral lichen planus |
|
|
| Pemphigoid |
|
|
| Pemphigus |
|
|
| Lupus erythematosus |
|
|
| Desquamative gingivitis |
|
|
| Drug-influenced gingival enlargement |
|
|
| Primary herpetic gingivostomatitis |
|
|
| Allergic reactions |
|
|
| Leukemia |
|
|
| Gingival candidosis |
|
|
| Primary and metastatic carcinoma | *Most gingival carcinomas, both primary and metastatic, present with localized exophytic masses rather than diffuse pseudoinflammatory changes generally associated with gingivitis. |
|
| Foreign body gingivitis |
|
|
| Orofacial granulomatosis |
|
|
| Pyostomatitis vegetans |
|
|
| Linear IgA disease |
|
|
| Wegener granulomatosis |
|
|
| Erythema multiforme |
|
|
| Agranulocytosis |
|
|
| Histoplasmosis |
|
|
| Cyclic neutropenia |
|
|
Epidemiology and Demographics
- Gingivitis is the commonest periodontal disease that is found to be more prevalent in males as compared to females as it has been found that females tend to follow better oral care regimes and thus in maintaining oral hygiene.
- Women: There have been studies that found gingivitis to be more prevalent in pregnant women as compared to non-pregnant women. Also, the severe form of gingivitis has been found to be predominant in pregnant women.
- It is commonly seen in children and adults and prevalent worldwide. .
- Young population: Gingivitis occurs in half the population by the age of 4 or 5 years, and the incidence continues to increase with age. The prevalence of gingivitis peaks at close to 100% at puberty, but after puberty it declines slightly and stays constant into adulthood. Some children exhibit severe gingivitis at puberty. Puberty-associated gingivitis is related to increases in steroid hormones.
- Socioeconomic status: Studies have found gingivitis to be more prevalent in people with low socioeconomic status as people with high socioeconomic status tend to show a more positive attitude towards the maintenance of oral hygiene. Also, they have better access to health care options.
Risk Factors
- Risk factor is defined as an environmental, behavioral, or biologic factor which directly increases the probability of a disease occurring and if absent or removed, reduces the disease probability.
| Modifiable risk factors | Non-modifiable risk factors |
|---|---|
|
|
Complications
- Recurrence of gingivitis
- Periodontitis
- Infection or abscess of the gingiva or the jaw bones
- Trench mouth (bacterial infection and ulceration of the gums)
Acute Necrotizing Ulcerative Gingitivitis (ANUG or Trench mouth)
- Chronic gingivitis can progress to ANUG if not treated and when the patient neglects oral hygiene completely or when the immune system of the patient is compromised.
- The condition is commonly seen in developing countries where the living conditions are poor.
- IT occurs most frequently in smokers and debilitated patients who are under stress. Other risk factors are poor oral hygiene, nutritional deficiencies, immunodeficiency (eg, HIV/AIDS, use of immunosuppressive drugs), and sleep deprivation. Some patients also have oral candidiasis.
- The aetiology of ANUG is the overgrowth of a particular type of pathogenic bacteria (fusiform-spirochete variety) but risk factors such as stress, poor nutrition and a compromised immune system can exacerbate the infection
- It is categorized under a severe form of gingivitis associated with pain, gingival bleeding, and ulceration. It is characterized by marked gingival edema, spontaneous bleeding, or bleeding in response to minimal local trauma. It may be associated with localized pain, altered taste (metallic taste mostly), and halitosis.
- Ulcerations, which are pathognomonic, are present on the dental papillae and marginal gingiva. These ulcerations have a characteristically punched-out appearance and are covered by a gray pseudomembrane. Similar lesions on the buccal mucosa and tonsils are rare. Swallowing and talking may be painful. Regional lymphadenopathy often is present.
- Treatment: It includes debridement, rinses (eg, hydrogen peroxide, chlorhexidine) and improved oral hygiene. If debridement is delayed (eg, if a dentist or the instruments necessary for debridement are unavailable), oral antibiotics (eg, amoxicillin 500 mg every 8 hours, erythromycin 250 mg every 6 hours, or tetracycline 250 mg every 6 hours) may help to provide relief and can be continued until 72 hours after symptoms resolve.
Prognosis
- Gingivitis, if identified and treated, can easily be resolved as the condition is reversible in its early stages.
- However, chronic gingivitis, if left untreated, can progress to periodontitis and can ultimately result in bone destruction, causing tooth loss.
Clinical presentation
- Onset: It can be acute or chronic, and can be either localized or generalized which is categorized as follows:
- Marginal gingivitis: An inflammation confined to the gingival margin.
- Papillary gingivitis: It involves interdental papillae.
- Diffuse gingivitis: It has diffuse involvement of the gingival margin, attached gingiva, and interdental papillae.
Clinical symptoms
The symptoms of gingivitis are as follows:
- Swollen gums
- Mouth sores
- Bright-red, or purple gums
- Shiny gums
- Gums that are painless, except when pressure is applied
- Gums that bleed easily, even with gentle brushing,and especially when you floss
- Gums that itch with varying degrees of severity
- Receding gumline
Clinical signs
| Medical condition | Clinical signs on examination |
|---|---|
| Bacterial dental biofilm only |
|
| Plaque-induced gingivitis | Clinical signs on examination |
| Puberty |
|
| Menstrual cycle |
|
| Pregnancy |
|
| Oral contraceptives |
|
| Hyperglycemia |
|
| Leukemia |
|
| Smoking |
|
| Malnutrition |
|
| Prominent subgingival restoration margins |
|
| Hyposalivation |
|
| Drug-influenced gingival enlargements |
|
Diagnosis
- A detailed history taking and physical examination (Table) should be performed.
- Clinical evaluation: Finding erythematous and friable tissue at the gum lines confirms the diagnosis of gingivitis. To detect early gingival disease, some dentists frequently measure the depth of the pocket around each tooth. Depths < 3 mm are normal; deeper pockets are at high risk of gingivitis and periodontitis.
- Laboratory test: Not routinely required.
- Radiographs: As gingivitis is a soft tissue disease, radiographic evaluation is not helpful. However, it should be done to rule out periodontitis or other differential disorder.
Treatment
- Treatment approach: An interprofessional approach is required to identify the causes of gingivitis and to intervene at an early stage before its progression to periodontitis.
- Aim: To restore the inflamed tissues to clinical health and then to maintain clinically healthy gingivae and subsequently preventing periodontitis. Periodontitis has also been linked to diabetes, arteriosclerosis, osteoporosis, pancreatic cancer and pre-term low birth weight babies.
- Stepwise approach:
- A dentist or dental hygienist will perform a thorough cleaning of the teeth and gums and remove localized factors initiating the inflammatory response. This includes scaling to thoroughly remove biofilm and deposits on the tooth structure and laser decontamination of the sulcus if possible. The removal of plaque is usually not painful, and the inflammation of the gums should be gone between one and two weeks.
- Ensure oral hygiene reinforcement by twice daily tooth brushing and once daily interdental cleaning (with an interdental brush or dental floss) and adjunctive chemical plaque control agents (such as chlorhexidine or essential oil-containing mouthwash).
- Address the modifiable systemic or local factors by changing the medication if drug induced; prescribing supplements in case of nutritional deficiency; and an indentification of faulty prosthesis should be done and replaced.
- In severe cases, patients can also be prescribed antibiotics.
Prevention
- Oral hygiene: Maintenance of a good oral hygiene can prevent the formation of plaque and gingivitis. Patients should be taught the correct brushing technique, frequency of brushing (twice daily) along with the use of floss.
- Brushing: Brushing after meals helps remove food debris and plaque trapped between your teeth and gums. Don’t forget to include your tongue, bacteria loves to hide there.
- Floss: Flossing at least once a day helps remove food particles and plaque between teeth and along the gum line that your toothbrush can’t quite reach.
- Swish with mouthwash: Mouthwash and gel containing antiseptic and anti-inflammatory properties can also be advised to the patient.
- Balanced diet: An importance of a balanced diet should be emphasized.
- Dentist visit: A routine cleaning by a dentist or hygienist at 6-month to 1-year intervals can help minimize gingivitis. Patients with systemic disorders predisposing to gingivitis require more frequent professional cleanings (from every 2 weeks to every 3 months).
- Know your risk: Age, smoking, diet and genetics can all increase your risk for periodontal disease. If you are at increased risk, be sure to talk with your dental professional.
References
External links
- Gingivitis - Medline plus
Template:Periodontology
Template:Oral pathology
zh-min-nan:Khí-hoāⁿ-iām
de:Gingivitis
el:Ουλίτιδα
it:Gengivite
nl:Tandvleesontsteking
simple:Gingivitis
fi:Ientulehdus
sv:Gingivit