Congenital adrenal hyperplasia Overview
|
Congenital adrenal hyperplasia main page |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Mehrian Jafarizade, M.D [2]
Synonyms and keywords: Congenital adrenal hyperplasia, CAH, Adrenal hyperplasia
Overview
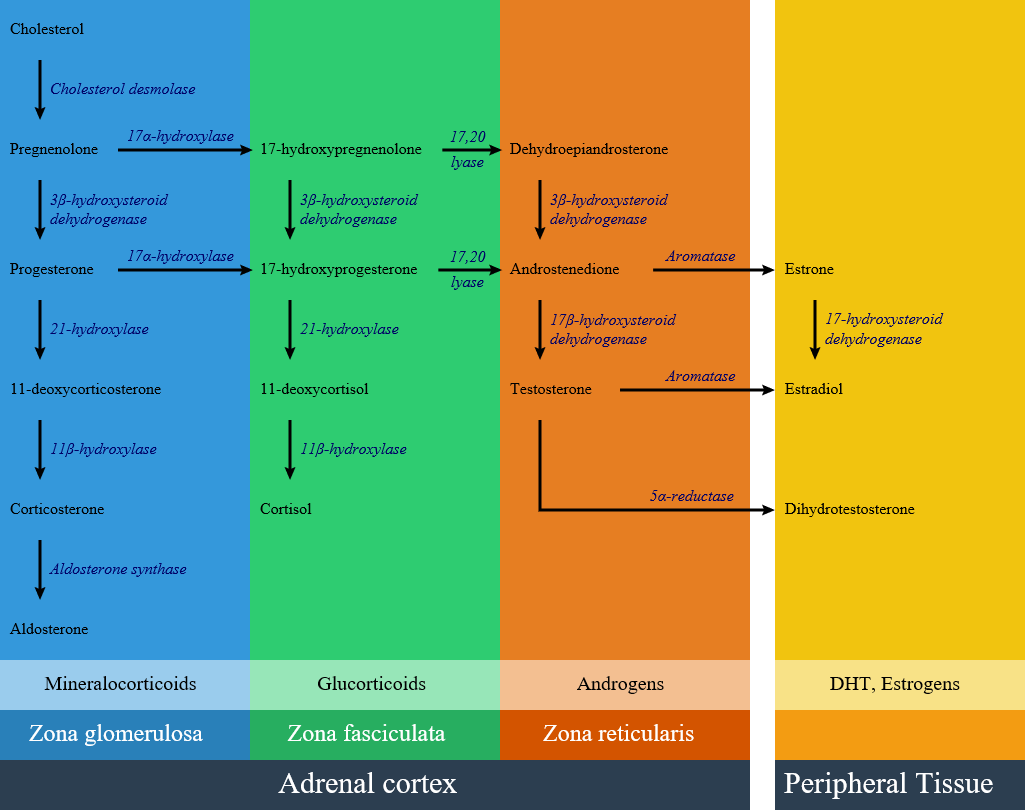
Congenital adrenal hyperplasia (CAH) refers to any of several autosomal recessive conditions resulting from biochemical paths of the steroidogenesis of cortisol from cholesterol by the adrenal glands. Most of these conditions involve greater or lesser production of sex steroids and can alter development of primary or secondary sex characteristics in affected infants, children, and adults. Only a small minority of people with CAH can be said to have an intersex condition, but this attracted American public attention in the late 1990s and many accounts of varying accuracy have appeared in the popular media. Approximately 95% of cases of CAH are due to 21-hydroxylase deficiency. Prenatal diagnosis can be made in both of these disorders by chorionic villous sampling, but this can only be done at 8-10 weeks. In order to prevent the deleterious effect of excess androgens on genital (and brain!) development, therapy must be started earlier. This is most often considered if there is an affected sibling. Treatment is dexamethasone, which is not degraded by the placenta, but is associated with significant maternal weight gain, hypertension, and edema.
Historical Perspective
- Congenital adrenal hyperplasia first time seen in 1865 by Luigi De Crecchio, an Italian pathologist, in a man at autopsy, who had large adrenal glands and female internal organs.
- Important aspects of discovering adrenal hormones:[1][2][3][4]
- In 1563, Eustachius described the adrenals and then published by Lancisi in 1714.
- In 1849, Thomas Addison, found on a bronzed appearance associated with the adrenal glands called melasma suprarenale while searching for the cause of pernicious anemia.
- In 1855, Thomas Addison defined the clinical features and autopsy findings in 11 cases of diseases of the suprarenal capsules, and half of them were tuberculous in origin.
- In 1856, In adrenalectomy experiments, Brown-Séquard found that the adrenal glands are nessesary for life.
- In 1896, William Osler prepared an oral glycerin extract derived from pig adrenals and showed that it had clinical benefit in patients with Addison disease.
- In 1905, Bulloch and Sequeira described patients with congenital adrenal hyperplasia.
- In 1936, Selye described the concept of stress and its effect on pituitary-adrenal function.
- In 1937-1952, Kendall and Reichstein, defined the isolation and structural characterization of adrenocortical hormones.
- In 1943, Li and colleagues isolated adrenocorticotropic hormone from sheep pituitary.
- In 1950, Hench, Kendall, and Reichstein shared the Nobel Prize in Medicine for describing the anti-inflammatory effects of cortisone in patients with rheumatoid arthritis
- In 1956, Conn described primary aldosteronism.
- In 1981, Vale defined characterization and synthesis of corticotropin-releasing hormone.
- From 1980-present called the molecular era; highlights in this section are:
- Cloning and functional characterization of steroid hormone receptors discovered.
- Steroidogenic enzymes described.
- Adrenal transcription factors were reported.
- Molecular basis for human adrenal diseases described.
Pathophisiology
Classification
Congenital adrenal hyperplasia is classified into seven types based on the genetic causes that lead to hyperplasia and hormonal imbalance.
| Disease | History and symptoms | Laboratory findings | Defective gene | ||||
|---|---|---|---|---|---|---|---|
| Blood pressure | Genitalia | Increased | Decreased | K levels | |||
| 21-hydroxylase deficiency | Classic type |
|
|
|
|
| |
| Non-classic type |
|
|
response to ACTH |
|
| ||
| 17-α hydroxylase deficiency |
|
|
|
| |||
| 11-β hydroxylase deficiency |
|
|
|
|
| ||
| 3β-Hydroxysteroid Dehydrogenase |
|
||||||
| Cytochrome P450-oxidoreductase (POR) deficiency (ORD) | |||||||
| Congenital lipoid adrenal hyperplasia | |||||||
| Cholesterol side-chain cleavage enzyme deficiency | |||||||
Prevention
References
- ↑ Delle Piane L, Rinaudo PF, Miller WL (2015). "150 years of congenital adrenal hyperplasia: translation and commentary of De Crecchio's classic paper from 1865". Endocrinology. 156 (4): 1210–7. doi:10.1210/en.2014-1879. PMID 25635623.
- ↑ Melmed, Shlomo (2016). Williams textbook of endocrinology. Philadelphia, PA: Elsevier. ISBN 978-0323297387.=
- ↑ HENCH PS, KENDALL EC (1949). "The effect of a hormone of the adrenal cortex (17-hydroxy-11-dehydrocorticosterone; compound E) and of pituitary adrenocorticotropic hormone on rheumatoid arthritis". Proc Staff Meet Mayo Clin. 24 (8): 181–97. PMID 18118071.
- ↑ Biglieri EG, Herron MA, Brust N (1966). "17-hydroxylation deficiency in man". J. Clin. Invest. 45 (12): 1946–54. doi:10.1172/JCI105499. PMC 292880. PMID 4288776.