Chronic renal failure pathophysiology: Difference between revisions
Feham Tariq (talk | contribs) |
Feham Tariq (talk | contribs) |
||
| Line 57: | Line 57: | ||
*The association between high concentrations of uremic solutes and adverse clinical outcomes is still controversial and is currently under investigation.<ref name="pmid16317811">{{cite journal| author=Palevsky PM, O'Connor T, Zhang JH, Star RA, Smith MW| title=Design of the VA/NIH Acute Renal Failure Trial Network (ATN) Study: intensive versus conventional renal support in acute renal failure. | journal=Clin Trials | year= 2005 | volume= 2 | issue= 5 | pages= 423-35 | pmid=16317811 | doi= | pmc=PMC1351394 | url=http://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=16317811 }} </ref><ref name="pmid16441870">{{cite journal| author=Canaud B, Morena M, Leray-Moragues H, Chalabi L, Cristol JP| title=Overview of clinical studies in hemodiafiltration: what do we need now ? | journal=Hemodial Int | year= 2006 | volume= 10 Suppl 1 | issue= | pages= S5-S12 | pmid=16441870 | doi=10.1111/j.1542-4758.2006.01183.x | pmc= | url=http://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=16441870 }} </ref> | *The association between high concentrations of uremic solutes and adverse clinical outcomes is still controversial and is currently under investigation.<ref name="pmid16317811">{{cite journal| author=Palevsky PM, O'Connor T, Zhang JH, Star RA, Smith MW| title=Design of the VA/NIH Acute Renal Failure Trial Network (ATN) Study: intensive versus conventional renal support in acute renal failure. | journal=Clin Trials | year= 2005 | volume= 2 | issue= 5 | pages= 423-35 | pmid=16317811 | doi= | pmc=PMC1351394 | url=http://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=16317811 }} </ref><ref name="pmid16441870">{{cite journal| author=Canaud B, Morena M, Leray-Moragues H, Chalabi L, Cristol JP| title=Overview of clinical studies in hemodiafiltration: what do we need now ? | journal=Hemodial Int | year= 2006 | volume= 10 Suppl 1 | issue= | pages= S5-S12 | pmid=16441870 | doi=10.1111/j.1542-4758.2006.01183.x | pmc= | url=http://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=16441870 }} </ref> | ||
*Although uremia is classically associated with chronic kidney failure, experimental studies have recently demonstrated a role of uremic solutes in [[acute kidney injury]] ([[acute uremia]]), but the pathophysiological significance of these solutes in the context of acute kidney injury is yet to be identified.<ref name="pmid19708999">{{cite journal| author=Herget-Rosenthal S, Glorieux G, Jankowski J, Jankowski V| title=Uremic toxins in acute kidney injury. | journal=Semin Dial | year= 2009 | volume= 22 | issue= 4 | pages= 445-8 | pmid=19708999 | doi=10.1111/j.1525-139X.2009.00598.x | pmc= | url=http://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=19708999 }} </ref> | *Although uremia is classically associated with chronic kidney failure, experimental studies have recently demonstrated a role of uremic solutes in [[acute kidney injury]] ([[acute uremia]]), but the pathophysiological significance of these solutes in the context of acute kidney injury is yet to be identified.<ref name="pmid19708999">{{cite journal| author=Herget-Rosenthal S, Glorieux G, Jankowski J, Jankowski V| title=Uremic toxins in acute kidney injury. | journal=Semin Dial | year= 2009 | volume= 22 | issue= 4 | pages= 445-8 | pmid=19708999 | doi=10.1111/j.1525-139X.2009.00598.x | pmc= | url=http://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=19708999 }} </ref> | ||
==References== | ==References== | ||
Revision as of 19:55, 1 June 2018
|
Chronic renal failure Microchapters |
|
Diagnosis |
|---|
|
Treatment |
|
Case Studies |
|
Chronic renal failure pathophysiology On the Web |
|
American Roentgen Ray Society Images of Chronic renal failure pathophysiology |
|
Risk calculators and risk factors for Chronic renal failure pathophysiology |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1] Associate Editor(s)-in-Chief: Aarti Narayan, M.B.B.S [2]Feham Tariq, MD [3]
Overview
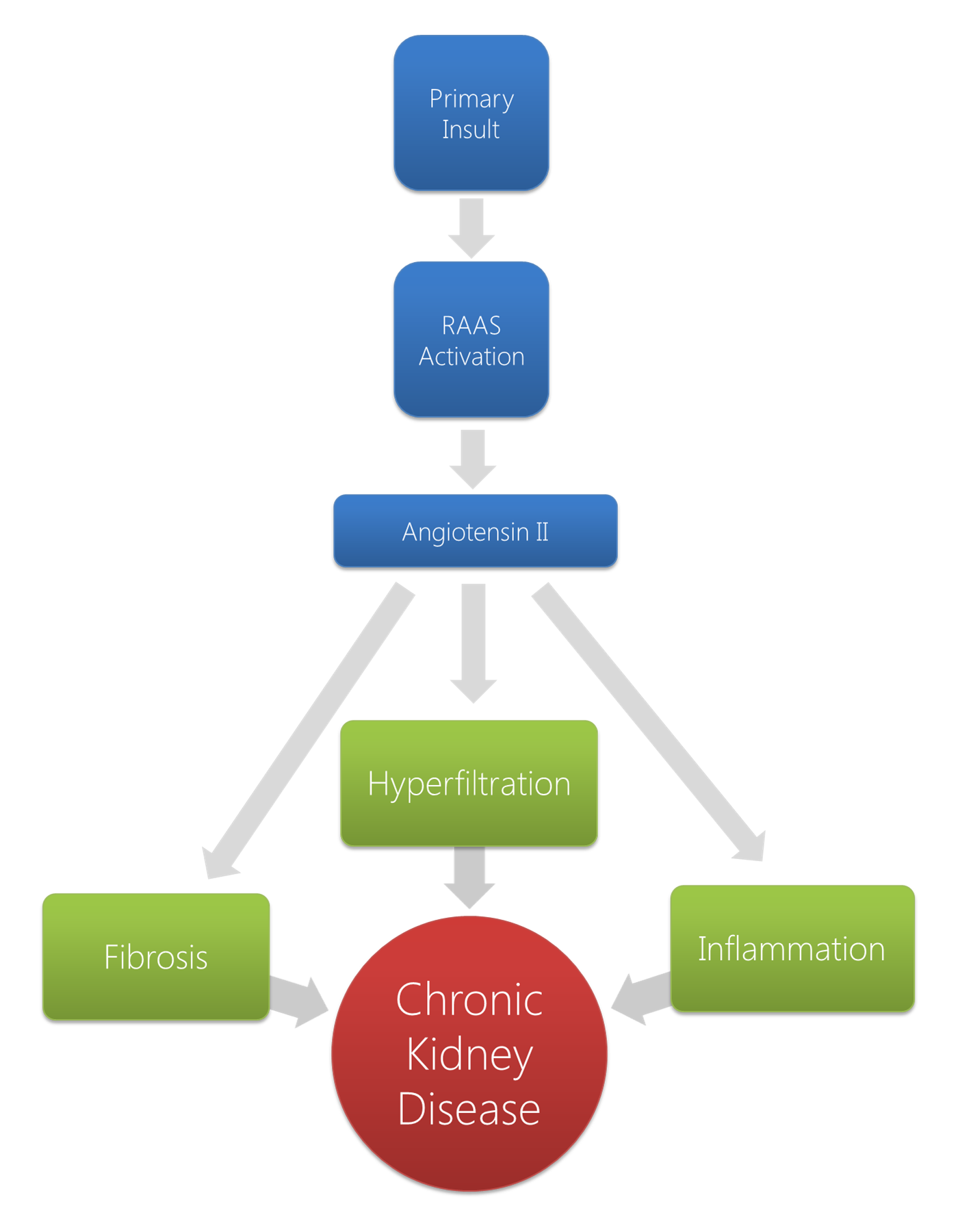
The pathophysiologic mechanisms leading to chronic kidney disease stem from the underlying etiologies responsible for the primary renal damage. Maladaptive systemic and renal responses arise that maintain and perpetuate the existing renal disease. Broadly, 3 main mechanisms exist related in part to the activation of the RAAS: hyperfiltration, inflammation, and accelerated fibrosis. As loss of kidney function progresses, nitrogen waste products are no longer cleared by the kidneys, and patients develop uremia as these uremic solutes accumulate over time.
Pathophysiology
The pathophysiologic mechanisms that lead to chronic kidney disease (CKD) stem from the underlying etiologies responsible for the primary renal damage. The initial insult is responsible for a decrease in the number of functional nephrons. However, beyond that initial insult, a form of maladaptive systemic and renal response arise that maintains and perpetuates the existing renal disease. With the activation of the renin-angiotensin-aldosterone system (RAAS), a combination of mechanisms herald a progressive loss of nephrons. Broadly, 3 main mechanisms exist related in part to the activation of the RAAS which are as follows:
- Hyperfiltration
- Inflammation
- Accelerated fibrosis
Hyperfiltration
The landmark works of Brenner et al were the first to propose the maladaptive changes that occur after renal injury. The team showed that after significant loss of nephron mass, major alterations in glomerular hemodynamics occur. The changes lead to glomerular hypertension with an increase in single nephron glomerular filtration rate termed hyperfiltration.[1] Hyperfiltration is a direct result of the increase in glomerular plasma flow and hydrostatic pressure in response to a decrease in preglomerular arteriolar resistance more than the decrease in postglomerular resistance with a net vasocontrictive effect on the efferent arteriole.[2]
The observed alterations occur due to the activation of the RAAS system. Initially, the juxtaglomerular apparatus increases the release of renin in response to the decreased perfusion pressure and solute delivery to the macula densa. Renin converts angiotensinogen to angiotensin I which is then converted to angiotensin II is then produced by angiotensin converting enzyme (ACE). Angiotensin II has been shown to be the main perpetrator in the maladaptation of the kidney to significant damage.[3]
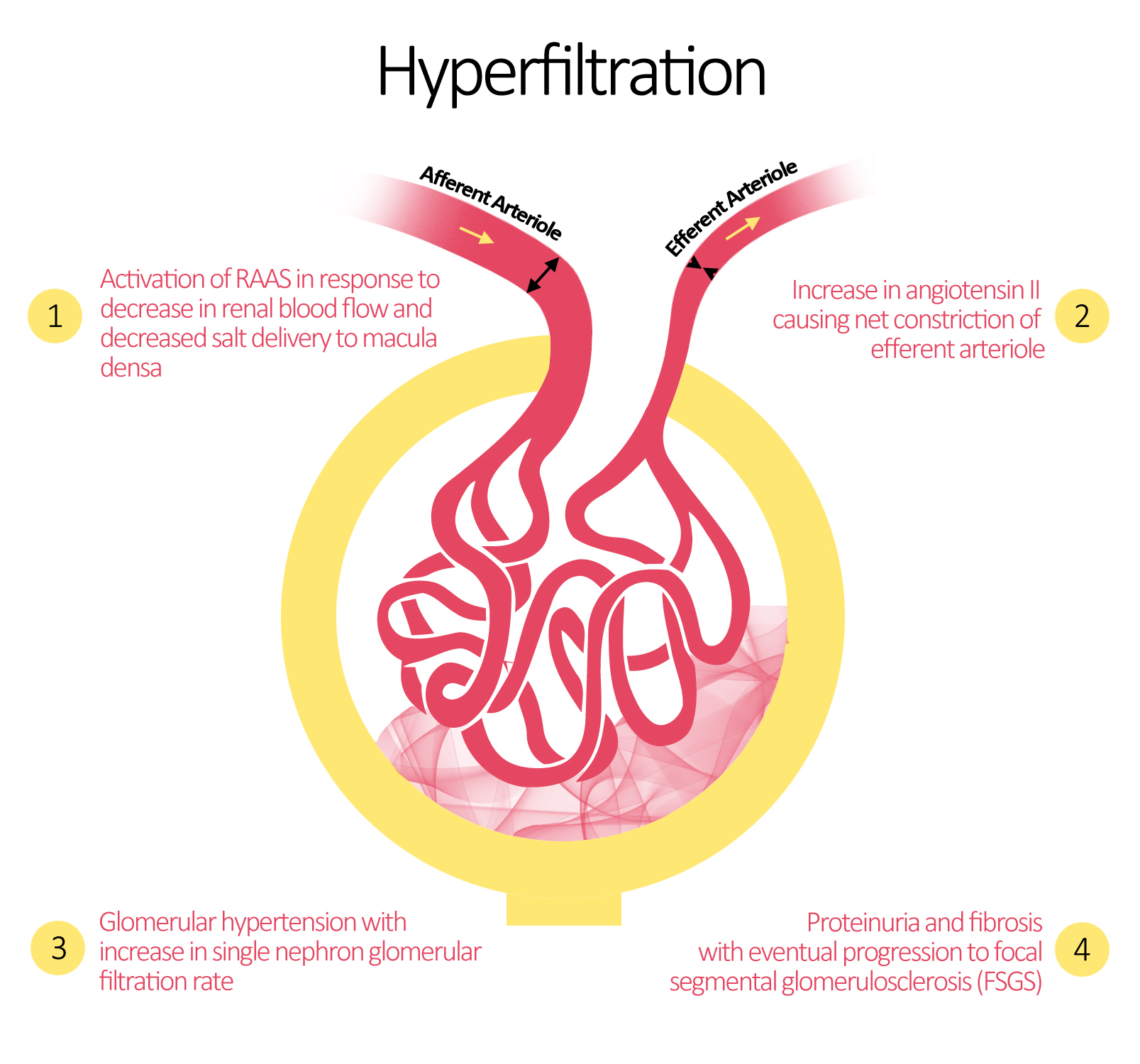
Most animal models exploring glomerular hypertension and hyperfiltration show progressive glomerular sclerosis and eventual proteinuria that usually occurs at a linear rate compared to the extent of nephron loss.[4][1][5][6] Furthermore, studies examining the prevention or reduction of glomerular hypertension and single nephron GFR have almost invariably shown a reduction in the rate of progression of renal disease.[7][8] Among the proposed interventions include dietary protein restriction, ACE inhibitors, and angiotensin receptor blockers (ARBs).[9]
Inflammation
Angiotensin II has also been linked to and increase in inflammation after renal injury. It has been shown to activate the transcription factor NF-κB, an important player in the inflammatory response mediating transcription of several cytokines and chemokines.[10] ATII has also been shown to stimulate endothelin-1 leading to the recruitment of T-cells and macrophages.[11] Beyond that, it upregulates the expression of adhesion molecules notably integrins, intracellular adhesion molecule-1, and vascular cellular adhesion molecule-1 all of which lead to and increase in leukocyte concentration in the area.[3] This creates a vicious cycle as lymphocytes can be a source of angiotensin II themselves amplifying its maladaptive effects.[12][13]
Accelerated Fibrosis
The increase in angiotensin II has also been directly associated with accelerated fibrosis in the remaining nephrons independently of the hemodynamic changes. Angiotensin II is thought to exert direct effects in the glomerular micromilieu leading to extracellular matrix (ECM) expansion. Angiotensin II has been shown to increase mRNA encoding type I procollagen and fibronectin in cultured mesangial cells. This effect is multiplied by the increase in expression of TGF-β further activating ECM protein production.[14] In normal renal tissue, the balance between ECM synthesis and degradation is essential to prevent fibrotic glomerular changes. Beyond the increase in ECM production, angiotensin II also disrupts this balance. Via ATI receptors, it activates tissue inhibitor of matrix metalloproteinases-1 (TIMP-1) and plasminogen activator inhibitor-1 (PAI-1) both of which shift the balance towards ECM accumulation.[3]
Another method of accelerated fibrosis is a process called epithelial-to-mesenchymal transition (EMT) where tissue epithelial cells transform into active fibroblasts. Although previously recognized as a physiologic mechanism during embryologic development, it has come to light as a process that provides fibroblasts during organ fibrosis after injury. Experimentally, more than one third of fibroblast at the site of renal injury were shown to originate from the renal tubular epithelial cells. The prototypical factor linked to EMT is TGF-β which is usually elevated after renal injury; however, other local factors also induce EMT including epidermal growth factor (EGF), Insulin growth factor II (IGF-II), and fibroblast growth factor (FGF-2).[15]
Uremia
Definition
Uremia (urine constituents in blood) is a clinical syndrome caused by progressive accumulation of nitrogen waste products among patients with kidney failure who with unable to clear these waste products by the kidneys.[16] It is thought to account for the clinical features of chronic kidney failure that cannot be explained by other classical abnormalities of chronic kidney failure (abnormalities of ion concentrations or extracellular volume overload).[16]
Progression to Uremia
- Uremia is a progressive clinical syndrome that is not typically characterized by a specific onset.[16]
- Progressive build-up of nitrogen waste products is usually detected among patients with eGFR below 50% of normal rate (normal GFR for a young healthy man approximately 100 to 120 mL/min/1.73m2).
- Clinical features of uremia are more evident with lower GFR values, and uremic features are often prominent as GFR drops below 10 ml/min/1.73m2 (loss of approximately 90% of kidney function), signaling the need for renal replacement modalities (either dialysis or transplantation)
Solutes of Uremia
- The majority of uremic solutes are unidentified.
- A few solutes that are present in high concentrations are identified. The toxic effects of these solutes have been studied.
- Examples of uremic solutes that have been studied include[16]:
- Beta-2-microglobulin
- Guanidines
- Nucleosides
- Phenols
- Indoles
- Furans
- Polyols
- Carbonyls
- Other advanced glycosylation end (AGE) products
- Protein intake is thought to increase the concentration of certain uremic solutes.
- Chemical characteristics of individual solutes may determine the capacity of dialysis to appropriately filter these solutes. Large solutes, solutes bound to albumin, and sequestered solutes are generally poorly filtered by conventional dialysis techniques. The use of ultrafiltration methods and improvement in dialysis membrane sizes are active areas of research that aim to increase filtration capacity of toxic solutes.[17][18]
- The association between high concentrations of uremic solutes and adverse clinical outcomes is still controversial and is currently under investigation.[19][18]
- Although uremia is classically associated with chronic kidney failure, experimental studies have recently demonstrated a role of uremic solutes in acute kidney injury (acute uremia), but the pathophysiological significance of these solutes in the context of acute kidney injury is yet to be identified.[20]
References
- ↑ 1.0 1.1 Brenner BM, Meyer TW, Hostetter TH (1982). "Dietary protein intake and the progressive nature of kidney disease: the role of hemodynamically mediated glomerular injury in the pathogenesis of progressive glomerular sclerosis in aging, renal ablation, and intrinsic renal disease". N Engl J Med. 307 (11): 652–9. doi:10.1056/NEJM198209093071104. PMID 7050706.
- ↑ Brenner BM, Lawler EV, Mackenzie HS (1996). "The hyperfiltration theory: a paradigm shift in nephrology". Kidney Int. 49 (6): 1774–7. PMID 8743495 Check
|pmid=value (help). - ↑ 3.0 3.1 3.2 Rüster C, Wolf G (2006). "Renin-angiotensin-aldosterone system and progression of renal disease". J Am Soc Nephrol. 17 (11): 2985–91. doi:10.1681/ASN.2006040356. PMID 17035613.
- ↑ Hostetter TH, Olson JL, Rennke HG, Venkatachalam MA, Brenner BM (1981). "Hyperfiltration in remnant nephrons: a potentially adverse response to renal ablation". Am J Physiol. 241 (1): F85–93. PMID 7246778.
- ↑ Fogo AB (2000). "Glomerular hypertension, abnormal glomerular growth, and progression of renal diseases". Kidney Int Suppl. 75: S15–21. PMID 10828756 Check
|pmid=value (help). - ↑ Hostetter TH, Rennke HG, Brenner BM (1982). "The case for intrarenal hypertension in the initiation and progression of diabetic and other glomerulopathies". Am J Med. 72 (3): 375–80. PMID 7036732.
- ↑ Anderson S, Meyer TW, Rennke HG, Brenner BM (1985). "Control of glomerular hypertension limits glomerular injury in rats with reduced renal mass". J Clin Invest. 76 (2): 612–9. doi:10.1172/JCI112013. PMC 423867. PMID 2993362.
- ↑ Meyer TW, Anderson S, Rennke HG, Brenner BM (1987). "Reversing glomerular hypertension stabilizes established glomerular injury". Kidney Int. 31 (3): 752–9. PMID 3033388.
- ↑ Wolf G, Ritz E (2005). "Combination therapy with ACE inhibitors and angiotensin II receptor blockers to halt progression of chronic renal disease: pathophysiology and indications". Kidney Int. 67 (3): 799–812. doi:10.1111/j.1523-1755.2005.00145.x. PMID 15698420.
- ↑ Wolf G, Wenzel U, Burns KD, Harris RC, Stahl RA, Thaiss F (2002). "Angiotensin II activates nuclear transcription factor-kappaB through AT1 and AT2 receptors". Kidney Int. 61 (6): 1986–95. doi:10.1046/j.1523-1755.2002.00365.x. PMID 12028439 Check
|pmid=value (help). - ↑ Hong HJ, Chan P, Liu JC, Juan SH, Huang MT, Lin JG; et al. (2004). "Angiotensin II induces endothelin-1 gene expression via extracellular signal-regulated kinase pathway in rat aortic smooth muscle cells". Cardiovasc Res. 61 (1): 159–68. PMID 14732213.
- ↑ Crowley SD, Frey CW, Gould SK, Griffiths R, Ruiz P, Burchette JL; et al. (2008). "Stimulation of lymphocyte responses by angiotensin II promotes kidney injury in hypertension". Am J Physiol Renal Physiol. 295 (2): F515–24. doi:10.1152/ajprenal.00527.2007. PMC 2519187. PMID 18495795.
- ↑ Suzuki Y, Ruiz-Ortega M, Lorenzo O, Ruperez M, Esteban V, Egido J (2003). "Inflammation and angiotensin II". Int J Biochem Cell Biol. 35 (6): 881–900. PMID 12676174.
- ↑ Wolf G (1998). "Link between angiotensin II and TGF-beta in the kidney". Miner Electrolyte Metab. 24 (2–3): 174–80. PMID 9525702 Check
|pmid=value (help). - ↑ Kalluri R, Neilson EG (2003). "Epithelial-mesenchymal transition and its implications for fibrosis". J Clin Invest. 112 (12): 1776–84. doi:10.1172/JCI20530. PMC 297008. PMID 14679171.
- ↑ 16.0 16.1 16.2 16.3 Meyer TW, Hostetter TH (2007). "Uremia". N Engl J Med. 357 (13): 1316–25. doi:10.1056/NEJMra071313. PMID 17898101.
- ↑ Eknoyan G, Beck GJ, Cheung AK, Daugirdas JT, Greene T, Kusek JW; et al. (2002). "Effect of dialysis dose and membrane flux in maintenance hemodialysis". N Engl J Med. 347 (25): 2010–9. doi:10.1056/NEJMoa021583. PMID 12490682.
- ↑ 18.0 18.1 Canaud B, Morena M, Leray-Moragues H, Chalabi L, Cristol JP (2006). "Overview of clinical studies in hemodiafiltration: what do we need now ?". Hemodial Int. 10 Suppl 1: S5–S12. doi:10.1111/j.1542-4758.2006.01183.x. PMID 16441870.
- ↑ Palevsky PM, O'Connor T, Zhang JH, Star RA, Smith MW (2005). "Design of the VA/NIH Acute Renal Failure Trial Network (ATN) Study: intensive versus conventional renal support in acute renal failure". Clin Trials. 2 (5): 423–35. PMC 1351394. PMID 16317811.
- ↑ Herget-Rosenthal S, Glorieux G, Jankowski J, Jankowski V (2009). "Uremic toxins in acute kidney injury". Semin Dial. 22 (4): 445–8. doi:10.1111/j.1525-139X.2009.00598.x. PMID 19708999.