Ceritinib
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Aparna Vuppala, M.B.B.S. [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Ceritinib is an antineoplastic agent that is FDA approved for the treatment of patients with anaplastic lymphoma kinase (ALK)-positive metastatic non-small cell lung cancer (NSCLC) who have progressed on or are intolerant to crizotinib. Common adverse reactions include increased ALT, nausea, increased AST, diarrhea , and vomiting and serious adverse drug reactions are convulsion, pneumonia, ILD/pneumonitis, dyspnea, dehydration, hyperglycemia, and nausea.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Non-small cell lung cancer
- Ceritinib is indicated for the treatment of patients with anaplastic lymphoma kinase (ALK)-positive metastatic non-small cell lung cancer (NSCLC) who have progressed on or are intolerant to crizotinib.
- This indication is approved under accelerated approval based on tumor response rate and duration of response . An improvement in survival or disease-related symptoms has not been established. Continued approval for this indication may be contingent upon verification and description of clinical benefit in confirmatory trials.
Dosing and Administration
- The recommended dose of Ceritinib is 750 mg orally once daily until disease progression or unacceptable toxicity. Administer Ceritinib on an empty stomach (i.e., do not administer within 2 hours of a meal) .
- A recommended dose has not been determined for patients with moderate to severe hepatic impairment.
- If a dose of Ceritinib is missed, make up that dose unless the next dose is due within 12 hours.
Dose Modifications for Adverse Reactions
- Recommendations for dose modifications of Ceritinib for adverse reactions are provided in Table 1.
- Approximately 60% of patients initiating treatment at the recommended dose required at least one dose reduction and the median time to first dose reduction was 7 weeks.
- Discontinue Ceritinib for patients unable to tolerate 300 mg daily.

Dose Modification for Strong CYP3A4 Inhibitors
- Avoid concurrent use of strong CYP3A inhibitors during treatment with Ceritinib
- If concomitant use of a strong CYP3A inhibitor is unavoidable, reduce the Ceritinib dose by approximately one-third, rounded to the nearest 150 mg dosage strength. After discontinuation of a strong CYP3A inhibitor, resume the Ceritinib dose that was taken prior to initiating the strong CYP3A4 inhibitor.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Ceritinib in adult patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Ceritinib in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
- The safety and effectiveness of Ceritinib in pediatric patients have not been established.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
- The safety and effectiveness of Ceritinib in pediatric patients have not been established.
Non–Guideline-Supported Use
- The safety and effectiveness of Ceritinib in pediatric patients have not been established.
Contraindications
- None
Warnings
Severe or Persistent Gastrointestinal Toxicity
- Diarrhea, nausea, vomiting, or abdominal pain occurred in 96% of 255 patients including severe cases in 14% of patients treated with Ceritinib in Study 1. Dose modification due to diarrhea, nausea, vomiting, or abdominal pain occurred in 38% of patients.
- Monitor and manage patients using standards of care, including anti-diarrheals, anti-emetics, or fluid replacement, as indicated. Based on the severity of the adverse drug reaction, withhold Ceritinib with resumption at a reduced dose as described in Table 1 .
Hepatotoxicity
- Drug-induced hepatotoxicity occurred in patients treated with Ceritinib . Elevations in alanine aminotransferase (ALT) greater than 5 times the upper limit of normal (ULN) occurred in 27% of 255 patients in Study 1. One patient (0.4%) required permanent discontinuation due to elevated transaminases and jaundice.
- Monitor with liver laboratory tests including ALT, aspartate aminotransferase (AST), and total bilirubin once a month and as clinically indicated, with more frequent testing in patients who develop transaminase elevations. Based on the severity of the adverse drug reaction, withhold Ceritinib with resumption at a reduced dose, or permanently discontinue Ceritinib as described in Table 1
Interstitial Lung Disease (ILD)/Pneumonitis
- Severe, life-threatening, or fatal ILD/pneumonitis can occur in patients treated with Ceritinib . In Study 1, pneumonitis was reported in 4% of 255 patients treated with Ceritinib . CTCAE Grade 3 or 4 ILD/pneumonitis was reported in 3% of patients, and fatal ILD/pneumonitis was reported in 1 patient (0.4%) in Study 1. One percent (1%) of patients discontinued Ceritinib in Study 1 due to ILD/pneumonitis.
- Monitor patients for pulmonary symptoms indicative of ILD/pneumonitis. Exclude other potential causes of ILD/pneumonitis, and permanently discontinue Ceritinib in patients diagnosed with treatment-related ILD/pneumonitis
QT Interval Prolongation
- QTc interval prolongation occurs in patients treated with Ceritinib . Three percent (3%) of 255 patients experienced a QTc interval increase over baseline greater than 60 msec in Study 1. Across the development program of Ceritinib , one of 304 patients (less than 1%) treated with Ceritinib doses ranging from 50 to 750 mg was found to have a QTc greater than 500 msec and 3% of patients had an increase from baseline QTc greater than 60 msec. A pharmacokinetic analysis suggested that Ceritinib causes concentration-dependent increases in the QT interval.
- When possible, avoid use of Ceritinib in patients with congenital long QT syndrome. Conduct periodic monitoring with electrocardiograms (ECGs) and electrolytes in patients with congestive heart failure, bradyarrhythmias, electrolyte abnormalities, or those who are taking medications that are known to prolong the QTc interval. Withhold Ceritinib in patients who develop a QTc interval greater than 500 msec on at least 2 separate ECGs until the QTc interval is less than 481 msec or recovery to baseline if the QTc interval is greater than or equal to 481 msec, then resume Ceritinib at a reduced dose as described in Table 1. Permanently discontinue Ceritinib in patients who develop QTc interval prolongation in combination with Torsade de pointes or polymorphic ventricular tachycardia or signs/symptoms of serious arrhythmia.
Hyperglycemia
- Hyperglycemia can occur in patients receiving Ceritinib . In Study 1, CTCAE Grade 3–4 hyperglycemia, based on laboratory values, occurred in 13% of 255 patients. There was a 6-fold increase in the risk of CTCAE Grade 3–4 hyperglycemia in patients with diabetes or glucose intolerance and a 2-fold increase in patients taking corticosteroids.
- Monitor serum glucose levels as clinically indicated. Initiate or optimize anti-hyperglycemic medications as indicated. Based on the severity of the adverse drug reaction, withhold Ceritinib until hyperglycemia is adequately controlled, then resume Ceritinib at a reduced dose as described in Table 1. If adequate hyperglycemic control cannot be achieved with optimal medical management, permanently discontinue Ceritinib .
Bradycardia
- Bradycardia can occur in patients receiving Ceritinib . In Study 1, sinus bradycardia, defined as a heart rate of less than 50 beats per minute, was noted as a new finding in 1% of 255 patients. Bradycardia was reported as an adverse drug reaction in 3% of patients in Study 1.
- Avoid using Ceritinib in combination with other agents known to cause bradycardia (e.g., beta-blockers, non-dihydropyridine calcium channel blockers, clonidine, and digoxin) to the extent possible. Monitor heart rate and blood pressure regularly. In cases of symptomatic bradycardia that is not life-threatening, withhold Ceritinib until recovery to asymptomatic bradycardia or to a heart rate of 60 bpm or above, evaluate the use of concomitant medications, and adjust the dose of Ceritinib . Permanently discontinue Ceritinib for life-threatening bradycardia if no contributing concomitant medication is identified; however, if associated with a concomitant medication known to cause bradycardia or hypotension, withhold Ceritinib until recovery to asymptomatic bradycardia or to a heart rate of 60 bpm or above, and if the concomitant medication can be adjusted or discontinued, resume Ceritinib at a reduced dose as described in Table 1 upon recovery to asymptomatic bradycardia or to a heart rate of 60 bpm or above, with frequent monitoring .
Embryofetal Toxicity
- Based on its mechanism of action, Ceritinib may cause fetal harm when administered to a pregnant woman. In animal studies, administration of ceritinib to rats and rabbits during organogenesis at maternal plasma exposures below the recommended human dose of 750 mg daily caused increases in skeletal anomalies in rats and rabbits. Apprise women of reproductive potential of the potential hazard to a fetus . Advise females of reproductive potential to use effective contraception during treatment with Ceritinib and for at least 2 weeks following completion of therapy
Adverse Reactions
Clinical Trials Experience
- The following adverse reactions are discussed in greater detail in other sections of the labeling:
- Severe or Persistent Gastrointestinal Toxicity
- Hepatotoxicity
- Interstitial Lung Disease/Pneumonitis
- QT Interval Prolongation
- Hyperglycemia
- Bradycardia
Clinical Trials Experience
- Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
- The safety evaluation of Ceritinib is based on 255 ALK-positive patients in Study 1 (246 patients with NSCLC and 9 patients with other cancers who received Ceritinib at a dose of 750 mg daily). The median duration of exposure to Ceritinib was 6 months. The study population characteristics were: median age 53 years, age less than 65 (84%), female (53%), Caucasian (63%), Asian (34%), NSCLC adenocarcinoma histology (90%), never or former smoker (97%), ECOG PS 0 or 1 (89%), brain metastasis (49%), and number of prior therapies 2 or more (67%).
- Dose reductions due to adverse reactions occurred in 59% of patients treated with Ceritinib . The most frequent adverse reactions, reported in at least 10% of patients, that led to dose reductions or interruptions were: increased ALT (29%), nausea (20%), increased AST (16%), diarrhea (16%), and vomiting (16%). Serious adverse drug reactions reported in 2% or more of patients in Study 1 were convulsion, pneumonia, ILD/pneumonitis, dyspnea, dehydration, hyperglycemia, and nausea. Fatal adverse reactions in patients treated with Ceritinib occurred in 5% of patients, consisting of: pneumonia (4 patients), respiratory failure, ILD/pneumonitis, pneumothorax, gastric hemorrhage, general physical health deterioration, pulmonary tuberculosis, cardiac tamponade, and sepsis (1 patient each). Discontinuation of therapy due to adverse reactions occurred in 10% of patients treated with Ceritinib . The most frequent adverse drug reactions that led to discontinuation in 1% or more of patients in Study 1 were pneumonia, ILD/pneumonitis, and decreased appetite.
- Tables 2 and 3 summarize the common adverse reactions and laboratory abnormalities observed in Ceritinib -treated patients.

- Additional clinically significant adverse reactions occurring in 2% or more of patients treated with Ceritinib included neuropathy (17%; comprised of paresthesia, muscular weakness, gait disturbance, peripheral neuropathy, hypoesthesia, peripheral sensory neuropathy, dysesthesia, neuralgia, peripheral motor neuropathy, hypotonia, or polyneuropathy), vision disorder (9%; comprised of vision impairment, blurred vision, photopsia, accommodation disorder, presbyopia, or reduced visual acuity), prolonged QT interval (4%), and bradycardia (3%).

Postmarketing Experience
There is limited information regarding Postmarketing Experience of Ceritinib in the drug label.
Drug Interactions
Effect of Other Drugs on Ceritinib
- Ceritinib is primarily metabolized by CYP3A4 and is a substrate of the efflux transporter P-glycoprotein (P-gp).
Strong CYP3A Inhibitors
- Ketoconazole (a strong CYP3A4/P-gp inhibitor) increased the systemic exposure of ceritinib . Avoid concurrent use of strong CYP3A inhibitors during treatment with Ceritinib . If concomitant use of strong CYP3A inhibitors including certain antivirals (e.g., ritonavir), macrolide antibiotics (e.g., telithromycin), antifungals (e.g., ketoconazole), and nefazodone is unavoidable, reduce the Ceritinib dose by approximately one-third, rounded to the nearest 150 mg dosage strength. After discontinuation of a strong CYP3A inhibitor, resume the Ceritinib dose that was taken prior to initiating the strong CYP3A4 inhibitor.
- Do not consume grapefruit and grapefruit juice as they may inhibit CYP3A.
Strong CYP3A Inducers
- Rifampin (a strong CYP3A4/P-gp inducer) decreased the systemic exposure of ceritinib . Avoid concurrent use of strong CYP3A inducers(e.g., carbamazepine, phenytoin, rifampin, and St. John’s Wort) during treatment with Ceritinib .
Effect of Ceritinib on Other Drugs
- Ceritinib may inhibit CYP3A and CYP2C9 at clinical concentrations. Avoid concurrent use of CYP3A and CYP2C9 substrates known to have narrow therapeutic indices or substrates primarily metabolized by CYP3A and CYP2C9 during treatment with Ceritinib . If use of these medications is unavoidable, consider dose reduction of CYP3A substrates with narrow therapeutic indices (e.g., alfentanil, cyclosporine, dihydroergotamine, ergotamine, fentanyl, pimozide, quinidine, sirolimus, tacrolimus) and CYP2C9 substrates with narrow therapeutic indices (e.g., phenytoin, warfarin).
Use in Specific Populations
Pregnancy
Risk Summary
- Based on its mechanism of action, Ceritinib may cause fetal harm when administered to a pregnant woman. In animal studies, administration of ceritinib to rats and rabbits during organogenesis at maternal plasma exposures below the recommended human dose caused increases in skeletal anomalies in rats and rabbits. If this drug is used during pregnancy, or if the patient becomes pregnant while taking this drug, apprise the patient of the potential hazard to a fetus.
Animal Data
- In an embryo-fetal development study in which pregnant rats were administered daily doses of ceritinib during organogenesis, dose-related skeletal anomalies were observed at doses as low as 50 mg/kg (less than 0.5-fold the human exposure by AUC at the recommended dose). Findings included delayed ossifications and skeletal variations.
- In pregnant rabbits administered ceritinib daily during organogenesis, dose-related skeletal anomalies, including incomplete ossification, were observed at doses equal to or greater than 2 mg/kg/day (approximately 0.015-fold the human exposure by AUC at the recommended dose). A low incidence of visceral anomalies, including absent or malpositioned gallbladder and retroesophageal subclavian cardiac artery, was observed at doses equal to or greater than 10 mg/kg/day (approximately 0.13-fold the human exposure by AUC at the recommended dose). Maternal toxicity and abortion occurred in rabbits at doses of 35 mg/kg or greater. In addition, embryolethality was observed in rabbits at a dose of 50 mg/kg.Risk Summary
- There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Ceritinib in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Ceritinib during labor and delivery.
Nursing Mothers
- It is not known whether ceritinib or its metabolites are present in human milk. Because many drugs are present in human milk and because of the potential for serious adverse reactions in nursing infants from ceritinib, advise mothers to discontinue nursing.
Pediatric Use
- The safety and effectiveness of Ceritinib in pediatric patients have not been established.
Geriatic Use
- Clinical studies of Ceritinib did not include sufficient numbers of subjects aged 65 years and older to determine whether they respond differently from younger subjects. Of the 255 patients in Study 1 who received Ceritinib at the recommended dose, 40 (16%) were 65 years or older.
Gender
There is no FDA guidance on the use of Ceritinib with respect to specific gender populations.
Race
There is no FDA guidance on the use of Ceritinib with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Ceritinib in patients with renal impairment.
Hepatic Impairment
- As ceritinib is eliminated primarily via the liver, patients with hepatic impairment may have increased exposure. Dose adjustment is not recommended for patients with |mild hepatic impairment (total bilirubin less than or equal to ULN and AST greater than ULN or total bilirubin greater than 1.0 to 1.5 times ULN and any AST) based on results of the population pharmacokinetic analysis . A recommended dose has not been determined for patients with moderate to severe hepatic impairment
Females of Reproductive Potential and Males
Contraception
- Based on its mechanism of action, Ceritinib may cause fetal harm when administered to a pregnant woman . Advise females of reproductive potential to use effective contraception during treatment with Ceritinib and for at least 2 weeks following completion of therapy.
Immunocompromised Patients
There is no FDA guidance one the use of Ceritinib in patients who are immunocompromised.
Administration and Monitoring
Administration
- Oral
Monitoring
- Monitor heart rate and blood pressure regularly. In cases of symptomatic bradycardia that is not life-threatening, withhold Ceritinib until recovery to asymptomatic bradycardia or to a heart rate of 60 bpm or above, evaluate the use of concomitant medications, and adjust the dose of Ceritinib .
- Monitor serum glucose levels as clinically indicated.
IV Compatibility
There is limited information regarding IV Compatibility of Ceritinib in the drug label.
Overdosage
There is limited information regarding Chronic Overdose of Ceritinib in the drug label.
Pharmacology

Mechanism of Action
- Ceritinib is a kinase inhibitor. Targets of ceritinib inhibition identified in either biochemical or cellular assays at clinically relevant concentrations include ALK, insulin-like growth factor 1 receptor (IGF-1R), insulin receptor (InsR), and ROS1. Among these, ceritinib is most active against ALK. Ceritinib inhibited autophosphorylation of ALK, ALK-mediated phosphorylation of the downstream signaling protein STAT3, and proliferation of ALK-dependent cancer cells in in vitro and in vivo assays.
- Ceritinib inhibited the in vitro proliferation of cell lines expressing EML4-ALK and NPM-ALK fusion proteins and demonstrated dose-dependent inhibition of EML4-ALK-positive NSCLC xenograft growth in mice and rats. Ceritinib exhibited dose-dependent anti-tumor activity in mice bearing EML4-ALK-positive NSCLC xenografts with demonstrated resistance to crizotinib, at concentrations within a clinically relevant range.
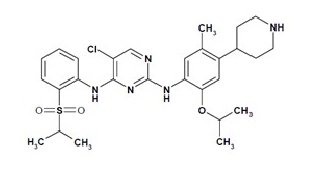
Structure
- Ceritinib (ceritinib) is a tyrosine kinase inhibitor for oral administration. The molecular formula for ceritinib is C28H36N5O3ClS. The molecular weight is 558.14 g/mole. Ceritinib is described chemically as 5-Chloro-N4-[2-[(1-methylethyl)sulfonyl]phenyl]-N2-[5-methyl-2-(1-methylethoxy)-4-(4-piperidinyl)phenyl]-2,4-pyrimidinediamine.
- The chemical structure of ceritinib is shown below:
- Ceritinib is a white to almost white or light yellow or light brown powder with a pKa of 9.7 and 4.1.
- Ceritinib is supplied as printed hard-gelatin capsules containing 150 mg of ceritinib and the following inactive ingredients: colloidal anhydrous silica, L-hydroxypropylcellulose, magnesium stearate, microcrystalline cellulose, sodium starch glycolate, and hard gelatin capsule shells. The capsule shell is composed of gelatin, indiogotine, and titanium dioxide.
Pharmacodynamics
Cardiac Electrophysiology
- Serial ECGs were collected following a single dose and at steady-state to evaluate the effect of ceritinib on the QT interval in an open-label, dose-escalation, and expansion study. A total of 304 patients were treated with Ceritinib doses ranging from 50 to 750 mg with 255 patients treated with Ceritinib 750 mg. One of 304 patients (less than 1%) was found to have a QTc greater than 500 msec and 10 patients (3%) had an increase from baseline QTc greater than 60 msec. A central tendency analysis of the QTc data at average steady-state concentrations demonstrated that the upper bound of the 2-sided 90% CI for QTc was 16 msec at Ceritinib 750 mg. A pharmacokinetic/pharmacodynamic analysis suggested concentration-dependent QTc interval prolongation .
- Based on central review of ECG data, 2 of 304 patients (0.7%) had bradycardia defined as less than 50 beats per minute. Bradycardia was reported as an adverse drug reaction in 3% of patients in Study 1.
Pharmacokinetics
Absorption
- After single oral administration of Ceritinib in patients, peak plasma levels (Cmax) of ceritinib were achieved at approximately 4 to 6 hours, and area under the curve (AUC) and Cmax increased dose proportionally over 50 to 750 mg. The absolute bioavailability of Ceritinib has not been determined.
- Following Ceritinib 750 mg once daily dosing, steady-state was reached by approximately 15 days with a geometric mean accumulation ratio of 6.2 after 3 weeks. Systemic exposure increased in a greater than dose proportional manner after repeat doses of 50 to 750 mg once daily.
- Systemic exposure of ceritinib was increased when administered with a meal. A food effect study conducted in healthy subjects with a single 500 mg ceritinib dose showed that a high‐fat meal (containing approximately 1000 calories and 58 grams of fat) increased ceritinib AUC by 73% and Cmaxby 41% and a low-fat meal (containing approximately 330 calories and 9 grams of fat) increased ceritinib AUC by 58% and Cmax by 43% as compared with the fasted state. A 400 mg or higher Ceritinib dose taken with a meal is expected to result in systemic exposure exceeding that of a 750 mg Ceritinib dose taken in the fasted state, and may increase adverse drug reactions.
Distribution
- Ceritinib is 97% bound to human plasma proteins, independent of drug concentration. The apparent volume of distribution (Vd/F) is 4230 L following a single 750 mg Ceritinib dose in patients. Ceritinib also has a slight preferential distribution to red blood cells, relative to plasma, with a mean in vitro blood-to-plasma ratio of 1.35.
Elimination
- Following a single 750 mg Ceritinib dose, the geometric mean apparent plasma terminal half-life (t1/2) of ceritinib was 41 hours in patients. Ceritinib demonstrates nonlinear PK over time. The geometric mean apparent clearance (CL/F) of ceritinib was lower at steady-state (33.2 L/h) after 750 mg daily dosing than after a single 750 mg dose (88.5 L/h).
- Metabolism: In vitro studies demonstrated that CYP3A was the major enzyme involved in the metabolic clearance of ceritinib. Following oral administration of a single 750 mg radiolabeled ceritinib dose, ceritinib as the parent compound was the main circulating component (82%) in human plasma.
- Excretion: Following oral administration of a single 750 mg radiolabeled ceritinib dose, 92.3% of the administered dose was recovered in the feces (with 68% as unchanged parent compound) while 1.3% of the administered dose was recovered in the urine.
Specific Populations
- Age, Gender, Race, and Body Weight: Age, gender, race, and body weight had no clinically important effect on the systemic exposure of ceritinib based on population pharmacokinetic analyses.
- Hepatic Impairment: As ceritinib is eliminated primarily via the liver, patients with hepatic impairment may have increased exposure. A pharmacokinetic trial in patients with hepatic impairment has not been conducted. Based on a population pharmacokinetic analysis of 48 patients with mild hepatic impairment (total bilirubin less than or equal to ULN and AST greater than ULN or total bilirubin greater than 1.0 to 1.5 times ULN and any AST) and 254 patients with normal hepatic function (total bilirubin less than or equal to ULN and AST less than or equal to ULN), ceritinib exposures were similar in patients with mild hepatic impairment and normal hepatic function. The pharmacokinetics of ceritinib has not been studied in patients with moderate to severe hepatic impairment .
- Renal Impairment: A pharmacokinetic trial in patients with renal impairment has not been conducted as ceritinib elimination via the kidney is low (1.3% of a single oral administered dose). Based on a population pharmacokinetic analysis of 97 patients with mild renal impairment (CLcr 60 to less than 90 mL/min), 22 patients with moderate renal impairment (CLcr 30 to less than 60 mL/min) and 183 patients with normal renal function (greater than or equal to 90 mL/min), ceritinib exposures were similar in patients with mild and moderate renal impairment and normal renal function. Patients with severe renal impairment (CLcr less than 30 mL/min) were not included in the clinical trial.
- Pediatrics: No trials have been conducted to evaluate the pharmacokinetics of ceritinib in pediatric patients.
Drug Interactions
- Effect of Strong CYP3A Inhibitors on Ceritinib: In vitro studies show that ceritinib is a substrate of CYP3A. Coadministration of a single 450 mg Ceritinib dose with ketoconazole (a strong CYP3A inhibitor) 200 mg twice daily for 14 days increased ceritinib AUC (90% CI) by 2.9-fold (2.5, 3.3) and Cmax (90% CI) by 22% (7%, 39%) in 19 healthy subjects . The steady-state AUC of ceritinib at reduced doses after coadministration with ketoconazole 200 mg twice daily for 14 days was predicted by simulations to be similar to the steady-state AUC of ceritinib alone .
- Effect of Strong CYP3A Inducers on Ceritinib: Coadministration of a single 750 mg Ceritinib dose with rifampin (a strong CYP3A inducer) 400 mg daily for 14 days decreased ceritinib AUC (90% CI) by 70% (61%, 77%) and Cmax (90% CI) by 44% (24%, 59%) in 19 healthy subjects.
- Effect of Ceritinib on CYP Substrates: Based on in vitro data, ceritinib may inhibit CYP3A and CYP2C9 at clinical concentrations. Time-dependent inhibition of CYP3A was also observed.
- Effect of Transporters on Ceritinib Disposition: Ceritinib is a substrate of efflux transporter P-gp, but is not a substrate of Breast Cancer Resistance Protein (BCRP), Multidrug Resistance Protein (MRP2), Organic Cation Transporter (OCT1), Organic Anion Transporter (OAT2), or Organic Anion Transporting Polypeptide (OATP1B1) in vitro. Drugs that inhibit P-gp may increase ceritinib concentrations.
- Effect of Ceritinib on Transporters: Based on in vitro data, ceritinib does not inhibit apical efflux transporters, P-gp, BCRP, or MRP2, hepatic uptake transporters OATP1B1 and OATP1B3, renal organic anion uptake transporters OAT1 and OAT3, or organic cation uptake transporters OCT1 and OCT2 at clinical concentrations.
- Effect of Gastric Acid Reducing Agents on Ceritinib: Gastric acid reducing agents (e.g., proton pump inhibitors, H2-receptor antagonists, antacids) may alter the solubility of ceritinib and reduce its bioavailability as ceritinib demonstrates pH-dependent solubility and becomes poorly soluble as pH increases in vitro. A dedicated study has not been conducted to evaluate the effect of gastric acid reducing agents on the bioavailability of ceritinib.
Nonclinical Toxicology
Carcinogenesis, Mutagenesis, Impairment of Fertility
- Carcinogenicity studies have not been performed with ceritinib.
- Ceritinib was not mutagenic in vitro in the bacterial reverse mutation (Ames) assay but induced numerical aberrations (aneugenic) in the in vitro cytogenetic assay using human lymphocytes, and micronuclei in the in vitro micronucleus test using TK6 cells. Ceritinib was not clastogenic in the in vivo rat micronucleus assay.
- There are no data on the effect of ceritinib on human fertility. Fertility/early embryonic development studies were not conducted with ceritinib. There were no adverse effects on male or female reproductive organs in general toxicology studies conducted in monkeys and rats at exposures equal to or greater than 0.5- and 1.5-fold, respectively, of the human exposure by AUC at the recommended dose of 750 mg.
Animal Toxicology and/or Pharmacology
- Target organs in nonclinical animal models included, but were not limited to, the pancreas, biliopancreatic/bile ducts, gastrointestinal tract, and liver. Pancreatic focal acinar cell atrophy was observed in rats at 1.5-fold the human exposure by AUC at the recommended dose. Biliopancreatic duct and bile duct necrosis was observed in rats at exposures equal to or greater than 5% of the human exposure by AUC at the recommended dose. Bile duct inflammation and vacuolation were also noted in monkeys at exposures equal to or greater than 0.5-fold the human exposure by AUC at the recommended dose. Frequent minimal necrosis and hemorrhage of the duodenum was exhibited in monkeys at 0.5-fold the human exposure by AUC, and in rats at an exposure similar to that observed clinically.
- Ceritinib crossed the blood brain barrier in rats with a brain-to-blood exposure (AUCinf) ratio of approximately 15%.
Clinical Studies
- The efficacy of Ceritinib was established in a multicenter, single-arm, open-label clinical trial (Study 1). A total of 163 patients with metastatic ALK-positive NSCLC who progressed while receiving or were intolerant to crizotinib were enrolled. All patients received Ceritinib at a dose of 750 mg once daily. The major efficacy outcome measure was objective response rate (ORR) according to RECIST v1.0 as evaluated by both investigators and a Blinded Independent Central Review Committee (BIRC). Duration of response (DOR) was an additional outcome measure.
- The study population characteristics were: median age 52 years, age less than 65 (87%), female (54%), Caucasian (66%), Asian (29%), never or former smoker (97%), ECOG PS 0 or 1 (87%), progression on previous crizotinib (91%), number of prior therapies 2 or more (84%), and adenocarcinoma histology (93%). Sites of extra-thoracic metastasis included brain (60%), liver (42%), and bone (42%). ALK-positivity was verified retrospectively by review of local test results for 99% of patients.
- Efficacy results from Study 1 are summarized in Table 4.

How Supplied
- Ceritinib 150 mg capsules
- Hard gelatin capsule with opaque blue cap and opaque white body; opaque blue cap marked in black ink with “LDK 150MG”, opaque white body marked in black ink with “NVR”. Available in:
- Bottles of 70 capsules NDC 0078-0640-70
Storage
- Store at 25°C (77°F); excursions permitted between 15°C to 30°C (59°F to 86°F) [see USP Controlled Room Temperature].
Images
Drug Images
{{#ask: Page Name::Ceritinib |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
{{#ask: Label Page::Ceritinib |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
- Advise the patient to read the FDA-approved patient labeling (Patient Information).
- Inform patients that diarrhea, nausea, vomiting, and abdominal pain are the most commonly reported adverse reactions in patients treated with Ceritinib . Inform patients of supportive care options such as anti-emetic and anti-diarrheal medications. Advise patients to contact their healthcare provider for severe or persistent gastrointestinal symptoms
- Inform patients of the signs and symptoms of hepatotoxicity. Advise patients to contact their healthcare provider immediately for signs or symptoms of hepatotoxicity
- Inform patients of the risks of severe or fatal ILD/pneumonitis. Advise patients to contact their healthcare provider immediately to report new or worsening respiratory symptoms
- Inform patients of the risks of QTc interval prolongation and bradycardia. Advise patients to contact their healthcare provider immediately to report new chest pain or discomfort, changes in heartbeat, palpitations, dizziness, lightheadedness, fainting, and changes in or new use of heart or blood pressure medications
- Inform patients of the signs and symptoms of hyperglycemia. Advise patients to contact their healthcare provider immediately for signs or symptoms of hyperglycemia
- Advise females to inform their healthcare provider if they are pregnant. Inform females of reproductive potential of the risk to a fetus. Advise females of reproductive potential to use effective contraception during treatment with Ceritinib and for at least 2 weeks following completion of therapy
- Advise females not to breastfeed during treatment with Ceritinib
- Inform patients not to consume grapefruit and grapefruit juice during treatment with Ceritinib .
- Take Ceritinib on an empty stomach (i.e., do not take within 2 hours of a meal) .
- Advise patients to make up a missed dose of Ceritinib unless the next dose is due within 12 hours
Precautions with Alcohol
- Alcohol-Ceritinib interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
- Zykadia®
Look-Alike Drug Names
- A® — B®
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
{{#subobject:
|Page Name=Ceritinib
|Pill Name=No image.jpg
|Drug Name=
|Pill Ingred=|+sep=;
|Pill Imprint=
|Pill Dosage={{{dosageValue}}} {{{dosageUnit}}}
|Pill Color=|+sep=;
|Pill Shape=
|Pill Size (mm)=
|Pill Scoring=
|Pill Image=
|Drug Author=
|NDC=
}}
{{#subobject:
|Label Page=Ceritinib |Label Name=Ceritinib06.png
}}
{{#subobject:
|Label Page=Ceritinib |Label Name=Ceritinib07.png
}}