Cefuroxime microbiology: Difference between revisions
(Created page with "__NOTOC__ {{Cefuroxime}} {{CMG}};{{AE}}{{AK}} Cefuroxime has in vitro activity against a wide range of gram-positive and gram-negative organisms, and it is highly stable in t...") |
No edit summary |
||
| Line 35: | Line 35: | ||
Reports from the laboratory giving results of the standard single-disk susceptibility test for organisms other than Haemophilus spp. and Neisseria gonorrhoeae with a 30-mcg cefuroxime disk should be interpreted according to the following criteria: | Reports from the laboratory giving results of the standard single-disk susceptibility test for organisms other than Haemophilus spp. and Neisseria gonorrhoeae with a 30-mcg cefuroxime disk should be interpreted according to the following criteria: | ||
[[image: | [[image:Cefor3.png]] | ||
Results for [[Haemophilus]] spp. should be interpreted according to the following criteria: | Results for [[Haemophilus]] spp. should be interpreted according to the following criteria: | ||
[[image: | [[image:Cefor4.png]] | ||
Results for [[Neisseria gonorrhoeae]] should be interpreted according to the following criteria: | Results for [[Neisseria gonorrhoeae]] should be interpreted according to the following criteria: | ||
[[image: | [[image:Cefor5.png]] | ||
Organisms should be tested with the cefuroxime disk since cefuroxime has been shown by in vitro tests to be active against certain strains found resistant when other beta-lactam disks are used. The cefuroxime disk should not be used for testing susceptibility to other cephalosporins. | Organisms should be tested with the cefuroxime disk since cefuroxime has been shown by in vitro tests to be active against certain strains found resistant when other beta-lactam disks are used. The cefuroxime disk should not be used for testing susceptibility to other cephalosporins. | ||
| Line 51: | Line 51: | ||
1. Testing for organisms other than Haemophilus spp. and Neisseria gonorrhoeae: | 1. Testing for organisms other than Haemophilus spp. and Neisseria gonorrhoeae: | ||
[[image: | [[image:Cefor6.png]] | ||
2. Testing for Haemophilus spp.: | 2. Testing for Haemophilus spp.: | ||
[[image: | [[image:Cefor7.png]] | ||
3. Testing for Neisseria gonorrhoeae: | 3. Testing for Neisseria gonorrhoeae: | ||
[[image: | [[image:Cefor8.png]] | ||
'''Dilution Techniques''' | '''Dilution Techniques''' | ||
Use a standardized dilution method1 (broth, agar, microdilution) or equivalent with cefuroxime powder. The MIC values obtained for bacterial isolates other than Haemophilus spp. and Neisseria gonorrhoeae should be interpreted according to the following criteria: | Use a standardized dilution method1 (broth, agar, microdilution) or equivalent with cefuroxime powder. The MIC values obtained for bacterial isolates other than Haemophilus spp. and Neisseria gonorrhoeae should be interpreted according to the following criteria: | ||
[[image: | [[image:Cefor9.png]] | ||
MIC values obtained for Haemophilus spp. should be interpreted according to the following criteria: | MIC values obtained for Haemophilus spp. should be interpreted according to the following criteria: | ||
[[image: | [[image:Cefor10.png]] | ||
MIC values obtained for Neisseria gonorrhoeae should be interpreted according to the following criteria: | MIC values obtained for Neisseria gonorrhoeae should be interpreted according to the following criteria: | ||
[[image: | [[image:Cefor11.png]] | ||
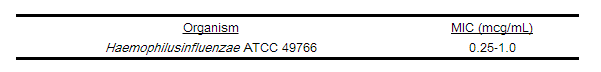
As with standard diffusion techniques, dilution methods require the use of laboratory control organisms. Standard cefuroxime powder should provide the following MIC values. | As with standard diffusion techniques, dilution methods require the use of laboratory control organisms. Standard cefuroxime powder should provide the following MIC values. | ||
| Line 78: | Line 78: | ||
1. For organisms other than Haemophilus spp. and Neisseria gonorrhoeae: | 1. For organisms other than Haemophilus spp. and Neisseria gonorrhoeae: | ||
[[image: | [[image:Cefor12.png]] | ||
2. For Haemophilus spp.: | 2. For Haemophilus spp.: | ||
[[image: | [[image:Cefor13.png]] | ||
3. For Neisseria gonorrhoeae: | 3. For Neisseria gonorrhoeae: | ||
[[image: | [[image:Cefor14.png]] | ||
Latest revision as of 02:34, 6 January 2014
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1];Associate Editor(s)-in-Chief: Abdurahman Khalil, M.D. [2]
Cefuroxime has in vitro activity against a wide range of gram-positive and gram-negative organisms, and it is highly stable in the presence of beta-lactamases of certain gram-negative bacteria. The bactericidal action of cefuroxime results from inhibition of cell-wall synthesis.
Cefuroxime is usually active against the following organisms in vitro.
Aerobes, Gram-positive
Staphylococcus aureus, Staphylococcus epidermidis, Streptococcus pneumoniae, and Streptococcus pyogenes (and other streptococci).
NOTE: Most strains of enterococci, e.g., Enterococcus faecalis (formerly Streptococcus faecalis), are resistant to cefuroxime. Methicillin-resistant staphylococci and Listeria monocytogenes are resistant to cefuroxime.
Aerobes, Gram-negative
Citrobacter spp., Enterobacter spp., Escherichia coli, Haemophilus influenzae (including ampicillin-resistant strains), Haemophilus parainfluenzae, Klebsiellaspp. (including Klebsiella pneumoniae), Moraxella(Branhamella) catarrhalis (including ampicillin- and cephalothin-resistant strains), Morganella morganii(formerly Proteus morganii), Neisseria gonorrhoeae (including penicillinase- and non–penicillinase-producing strains),Neisseria meningitidis, Proteus mirabilis, Providencia rettgeri(formerly Proteus rettgeri), Salmonella spp., andShigella spp.
NOTE: Some strains of Morganella morganii, Enterobacter cloacae, and Citrobacter spp. have been shown by in vitro tests to be resistant to cefuroxime and other cephalosporins. Pseudomonas and Campylobacter spp., Legionella spp.,Acinetobacter calcoaceticus, and most strains of Serratia spp. and Proteus vulgaris are resistant to most first- and second-generation cephalosporins.
Anaerobes
Gram-positive and gram-negative cocci (including Peptococcus and Peptostreptococcus spp.), gram-positive bacilli (including Clostridium spp.), and gram-negative bacilli (including Bacteroides and Fusobacterium spp.).
NOTE: Clostridium difficile and most strains of Bacteroides fragilis are resistant to cefuroxime.
Susceptibility Tests
Diffusion Techniques
Quantitative methods that require measurement of zone diameters give an estimate of antibiotic susceptibility. One such standard procedure1 that has been recommended for use with disks to test susceptibility of organisms to cefuroxime uses the 30-mcg cefuroxime disk. Interpretation involves the correlation of the diameters obtained in the disk test with the minimum inhibitory concentration (MIC) for cefuroxime.
A report of “Susceptible” indicates that the pathogen is likely to be inhibited by generally achievable blood levels. A report of “Moderately Susceptible” suggests that the organism would be susceptible if high dosage is used or if the infection is confined to tissues and fluids in which high antibiotic levels are attained. A report of “Intermediate” suggests an equivocable or indeterminate result. A report of “Resistant” indicates that achievable concentrations of the antibiotic are unlikely to be inhibitory and other therapy should be selected.
Reports from the laboratory giving results of the standard single-disk susceptibility test for organisms other than Haemophilus spp. and Neisseria gonorrhoeae with a 30-mcg cefuroxime disk should be interpreted according to the following criteria:
Results for Haemophilus spp. should be interpreted according to the following criteria:
Results for Neisseria gonorrhoeae should be interpreted according to the following criteria:
Organisms should be tested with the cefuroxime disk since cefuroxime has been shown by in vitro tests to be active against certain strains found resistant when other beta-lactam disks are used. The cefuroxime disk should not be used for testing susceptibility to other cephalosporins.
Standardized procedures require the use of laboratory control organisms. The 30-mcg cefuroxime disk should give the following zone diameters.
1. Testing for organisms other than Haemophilus spp. and Neisseria gonorrhoeae:
2. Testing for Haemophilus spp.:
3. Testing for Neisseria gonorrhoeae:
Dilution Techniques Use a standardized dilution method1 (broth, agar, microdilution) or equivalent with cefuroxime powder. The MIC values obtained for bacterial isolates other than Haemophilus spp. and Neisseria gonorrhoeae should be interpreted according to the following criteria:
MIC values obtained for Haemophilus spp. should be interpreted according to the following criteria:
MIC values obtained for Neisseria gonorrhoeae should be interpreted according to the following criteria:
As with standard diffusion techniques, dilution methods require the use of laboratory control organisms. Standard cefuroxime powder should provide the following MIC values.
1. For organisms other than Haemophilus spp. and Neisseria gonorrhoeae:
2. For Haemophilus spp.:
3. For Neisseria gonorrhoeae:
References
http://www.accessdata.fda.gov/drugsatfda_docs/label/2001/50643s11lbl.pdf