Cefuroxime direction for use
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1];Associate Editor(s)-in-Chief: Abdurahman Khalil, M.D. [2]
Instructions for Constitution of ADD-Vantage® Vials
To Open Diluent Container:
Peel the corner of the ADD-Vantage diluent overwrap and remove flexible diluent container. Some opacity of the plastic flexible container due to moisture absorption during the sterilization process may be observed. This is normal and does not affect the solution quality or safety. The opacity will diminish gradually.
To Assemble Vial and Flexible DiluentContainer(Use Aseptic Technique):
1. Remove the protective covers from the top of the vial and the vial port on the diluent container as follows:
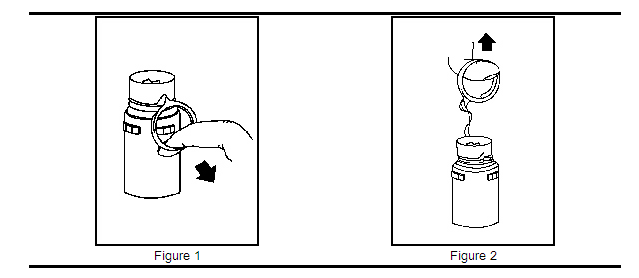
a. To remove the breakaway vial cap, swing the pull ring over the top of the vial and pull down far enough to start the opening (see Figure 1), then pull straight up to remove the cap (see Figure 2). Note: Once the breakaway cap has been removed, do not access vial with syringe.
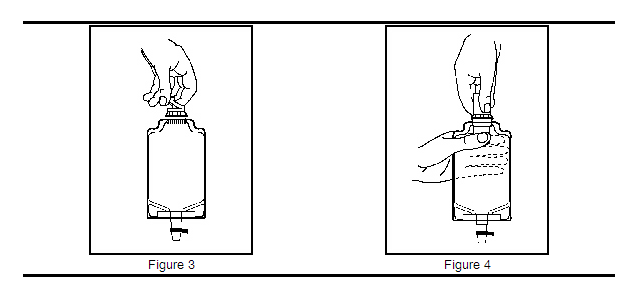
b. To remove the vial port cover, grasp the tab on the pull ring, pull up to break the 3 tie strings, then pull back to remove the cover (see Figure 3).
2. Screw the vial into the vial port until it will go no further. THE VIAL MUST BE SCREWED IN TIGHTLY TO ASSURE A SEAL. This occurs approximately one-half turn (180°) after the first audible click (see Figure 4). The clicking sound does not assure a seal; the vial must be turned as far as it will go. Note:Once vial is seated, do not attempt to remove (see Figure 4).
3. Recheck the vial to assure that it is tight by trying to turn it further in the direction of assembly.
4. Label appropriately.
To Prepare Admixture:
1. Squeeze the bottom of the diluent container gently to inflate the portion of the container surrounding the end of the drug vial.
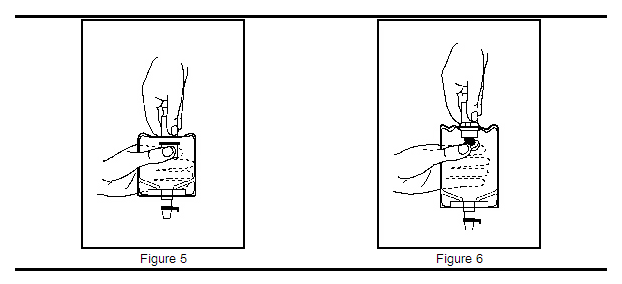
2. With the other hand, push the drug vial down into the container, telescoping the walls of the container. Grasp the inner cap of the vial through the walls of the container (see Figure 5).
3. Pull the inner cap from the drug vial (see Figure 6). Verify that the rubber stopper has been pulled out, allowing the drug and diluent to mix.
4. Mix container contents thoroughly and use within the specified time.
Preparation for Administration (Use Aseptic Technique):
1. Confirm the activation and admixture of vial contents.
2. Check for leaks by squeezing container firmly. If leaks are found, discard unit as sterility may be impaired.
3. Close flow control clamp of administration set.
4. Remove cover from outlet port at bottom of container.
5. Insert piercing pin of administration set into port with a twisting motion until the pin is firmly seated. Note: See full directions on administration set carton.
6. Lift the free end of the hanger loop on the bottom of the vial, breaking the 2 tie strings. Bend the loop outward to lock it in the upright position, then suspend container from hanger.
7. Squeeze and release drip chamber to establish proper fluid level in chamber.
8. Open flow control clamp and clear air from set. Close clamp.
9. Attach set to venipuncture device. If device is not indwelling, prime and make venipuncture.
10. Regulate rate of administration with flow control clamp.
WARNING: Do not use flexible container in series connections.
References
http://www.accessdata.fda.gov/drugsatfda_docs/label/2001/50643s11lbl.pdf