Cefotaxime sodium: Difference between revisions
No edit summary |
No edit summary |
||
| Line 224: | Line 224: | ||
In addition to the adverse reactions listed above which have been observed in patients treated with cefotaxime sodium, the following adverse reactions and altered laboratory tests have been reported for [[cephalosporin]] class antibiotics: allergic reactions, hepatic dysfunction including [[cholestasis]], [[aplastic anemia]], [[hemorrhage]], and false-positive test for urinary glucose. | In addition to the adverse reactions listed above which have been observed in patients treated with cefotaxime sodium, the following adverse reactions and altered laboratory tests have been reported for [[cephalosporin]] class antibiotics: allergic reactions, hepatic dysfunction including [[cholestasis]], [[aplastic anemia]], [[hemorrhage]], and false-positive test for urinary glucose. | ||
Several cephalosporins have been implicated in triggering [[seizures]], particularly in patients with [[renal impairment]] when the dosage was not reduced. If [[seizures]] associated with drug therapy occur, the drug should be discontinued. [[Anticonvulsant]] therapy can be given if clinically indicated. | Several cephalosporins have been implicated in triggering [[seizures]], particularly in patients with [[renal impairment]] when the dosage was not reduced. If [[seizures]] associated with drug therapy occur, the drug should be discontinued. [[Anticonvulsant]] therapy can be given if clinically indicated. | ||
|drugInteractions=*Increased [[nephrotoxicity]] has been reported following concomitant administration of [[cephalosporins]] and [[aminoglycoside]] [[antibiotics]]. | |||
*[[Probenecid]] interferes with the renal tubular transfer of cefotaxime, decreasing the total clearance of cefotaxime by approximately 50% and increasing the plasma concentrations of cefotaxime. Administration of cefotaxime in excess of 6 grams/day should be avoided in patients receiving [[probenecid]]. | |||
*A single intravenous dose and oral dose of probenecid (500 mg each) followed by two oral doses of probenecid 500 mg at approximately hourly intervals administered to three healthy male subjects receiving a continuous infusion of cefotaxime increased the steady-state plasma concentration of cefotaxime by approximately 80%. In another study, administration of oral probenecid 500 mg every 6 hours to six healthy male subjects with cefotaxime 1 gram infused over 5 minutes decreased the total clearance of cefotaxime by approximately 50%. | |||
*Additionally, no disulfiram-like reactions were reported in a study conducted in 22 healthy volunteers administered cefotaxime and ethanol. | |||
|FDAPregCat=B | |||
|useInPregnancyFDA=*Reproduction studies have been performed in pregnant mice given cefotaxime intravenously at doses up to 1200 mg/kg/day (0.4 times the recommended human dose based on mg/m2) or in pregnant rats when administered intravenously at doses up to 1200 mg/kg/day (0.8 times the recommended human dose based on mg/m2). | |||
*No evidence of embryotoxicity or [[teratogenicity]] was seen in these studies. *Although cefotaxime has been reported to cross the placental barrier and appear in cord blood, the effect on the human [[fetus]] is not known. | |||
*There are no well-controlled studies in pregnant women. Because animal reproductive studies are not always predictive of human response, this drug should be used during [[pregnancy]] only if clearly needed. | |||
|AUSPregCat=B1 | |||
|useInNursing=*Cefotaxime is excreted in human milk in low concentrations. | |||
*Caution should be exercised when cefotaxime is administered to a nursing woman. | |||
|useInGeri=*Of the 1409 subjects in clinical studies of cefotaxime, 632 (45%) were 65 and over, while 258 (18%) were 75 and over. | |||
*No overall differences in safety or effectiveness were observed between these subjects and younger subjects, and other reported clinical experience has not identified differences in responses between the elderly and younger patients, but greater sensitivity of some older individuals cannot be ruled out. | |||
|overdose=*The acute toxicity of cefotaxime was evaluated in neonatal and adult mice and rats. | |overdose=*The acute toxicity of cefotaxime was evaluated in neonatal and adult mice and rats. | ||
*Significant mortality was seen at parenteral doses in excess of 6000 mg/kg/day in all groups. | *Significant mortality was seen at parenteral doses in excess of 6000 mg/kg/day in all groups. | ||
| Line 232: | Line 246: | ||
*No specific antidote exists. | *No specific antidote exists. | ||
*Patients who receive an acute overdosage should be carefully observed and given supportive treatment. | *Patients who receive an acute overdosage should be carefully observed and given supportive treatment. | ||
| | |mechAction=Cefotaxime sodium is a bactericidal agent that acts by inhibition of bacterial cell wall synthesis. Cefotaxime has activity in the presence of some of beta-lactamases, both penicillinases and cephalosporinases, of Gram-negative and Gram-positive bacteria. | ||
===Mechanism of Resistance=== | |||
Resistance to cefotaxime is primarily through hydrolysis by beta-lactamase, alteration of penicillin-binding proteins (PBPs), and decreased permeability. | |||
|structure=Sterile cefotaxime sodium is a semisynthetic, broad spectrum cephalosporin antibiotic for parenteral administration. It is the sodium salt of 7-[2-(2-amino-4-thiazolyl) glyoxylamido]-3(hydroxymethyl)-8-oxo-5-thia-1-azabicyclo [4.2.0] oct-2-ene-2-carboxylate 72 (Z)-(o-methyloxime), acetate (ester). Cefotaxime for Injection, USP contains approximately 50.5 mg (2.2 mEq) of sodium per gram of cefotaxime activity. Solutions of Cefotaxime for Injection, USP range from very pale yellow to light amber depending on the concentration and the diluent used. The pH of the injectable solutions usually ranges from 5.0 to 7.5. | |||
[[File:Cefotaxime chemical structure.png|none|450px]] | |||
|alcohol=No disulfiram-like reactions were reported in a study conducted in 22 healthy volunteers administered cefotaxime and ethanol. | |||
|brandNames=*Claforan | |||
}} | }} | ||
Revision as of 16:30, 13 January 2015
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Stefano Giannoni [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Cefotaxime sodium is a 3rd Generation Cephalosporin that is FDA approved for the treatment of lower respiratory tract infections, genitourinary infections, gynecologic infections, bacteremia/septicemia, skin and skin structure infections, intra-abdominal infections, bone and/or joint infections and central nervous system infections. Common adverse reactions include {{{adverseReactions}}}.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Cefotaxime for Injection, USP is indicated for the treatment of patients with serious infections caused by susceptible strains of the designated microorganisms in the diseases listed below.
Lower respiratory tract infections
- Inncluding pneumonia, caused by:
- Streptococcus pneumoniae (formerly Diplococcus pneumoniae)
- Streptococcus pyogenes (Group A streptococci) and other streptococci (excluding enterococci, e.g., Enterococcus faecalis)
- Staphylococcus aureus (penicillinase and non-penicillinase producing)
- Escherichia coli
- Klebsiella species
- Haemophilus influenzae (including ampicillin resistant strains)
- Haemophilus parainfluenzae
- Proteus mirabilis
- Serratia marcescens
- Enterobacter species
- Indole positive Proteus and Pseudomonas species (including P. aeruginosa).
Genitourinary infections
Urinary tract infections
- caused by:
- Enterococcus species
- Staphylococcus epidermidis
- Staphylococcus aureus (penicillinase and non-penicillinase producing)
- Citrobacter species
- Enterobacter species
- Escherichia coli
- Klebsiella species
- Proteus mirabilis
- Proteus vulgaris
- Providencia stuartii
- Morganella morganii
- Providencia rettgeri
- Serratia marcescens
- Pseudomonas species (including P. aeruginosa).
Uncomplicated gonorrhea (cervical/urethral and rectal)
- Caused by:
- Neisseria gonorrhoeae, including penicillinase producing strains.
Gynecologic infections
Including:
- Pelvic inflammatory disease
- Endometritis
- Pelvic cellulitis
Caused by:
- Staphylococcus epidermidis
- Streptococcus species
- Enterococcus species
- Enterobacter species
- Klebsiella species
- Escherichia coli
- Proteus mirabilis
- Bacteroides species (including Bacteroides fragilis)
- Clostridium species
- Anaerobic cocci (including Peptostreptococcus species and Peptococcus species)
- Fusobacterium species (including F. nucleatum)
- Cefotaxime for Injection, USP, like other cephalosporins, has no activity against Chlamydia trachomatis. Therefore, when cephalosporins are used in the treatment of patients with pelvic inflammatory disease and C. trachomatis is one of the suspected pathogens, appropriate anti-chlamydial coverage should be added.
Bacteremia/Septicemia
- Caused by:
- Escherichia coli
- Klebsiella species
- Serratia marcescens
- Staphylococcus aureus
- Streptococcus species (including S. pneumoniae).
Skin and skin structure infections
- Caused by:
- Staphylococcus aureus (penicillinase and non-penicillinase producing)
- Staphylococcus epidermidis
- Streptococcus pyogenes (Group A streptococci) and other streptococci, **Enterococcus species
- Acinetobacter species
- Escherichia coli
- Citrobacter species (including C. freundii)
- Enterobacter species
- Klebsiella species
- Proteus mirabilis
- Proteus vulgaris
- Morganella morganii
- Providencia rettgeri
- Pseudomonas species
- Serratia marcescens
- Bacteroides species
- Anaerobic cocci (including Peptostreptococcus species and Peptococcus species).
Intra-abdominal infections
Including:
- Peritonitis:
Caused by:
- Streptococcus species
- Escherichia coli
- Klebsiella species
- Bacteroides species
- Anaerobic cocci (including Peptostreptococcus* species and Peptococcus* species)
- Proteus mirabilis
- Clostridium species
Bone and/or joint infections
Caused by:
- Staphylococcus aureus (penicillinase and non-penicillinase producing strains)
- Streptococcus species (including S. pyogenes)
- Pseudomonas species (including P. aeruginosa)
- Proteus mirabilis
Central nervous system infections
- Meningitis
- Ventriculitis
Caused by:
- Neisseria meningitidis
- Haemophilus influenzae
- Streptococcus pneumoniae
- Klebsiella pneumoniae
- Escherichia coli
Although many strains of enterococci (e.g., S. faecalis) and Pseudomonas species are resistant to cefotaxime sodium in vitro, Cefotaxime for Injection, USP has been used successfully in treating patients with infections caused by susceptible organisms.
Specimens for bacteriologic culture should be obtained prior to therapy in order to isolate and identify causative organisms and to determine their susceptibilities to cefotaxime. Therapy may be instituted before results of susceptibility studies are known; however, once these results become available, the antibiotic treatment should be adjusted accordingly.
In certain cases of confirmed or suspected gram-positive or gram-negative sepsis or in patients with other serious infections in which the causative organism has not been identified, Cefotaxime for Injection, USP may be used concomitantly with an aminoglycoside. The dosage recommended in the labeling of both antibiotics may be given and depends on the severity of the infection and the patient's condition. Renal function should be carefully monitored, especially if higher dosages of the aminoglycosides are to be administered or if therapy is prolonged, because of the potential nephrotoxicity and ototoxicity of aminoglycoside antibiotics. It is possible that nephrotoxicity may be potentiated if Cefotaxime for Injection, USP is used concomitantly with an aminoglycoside.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Cefotaxime sodium in adult patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Cefotaxime sodium in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
There is limited information regarding Cefotaxime sodium FDA-Labeled Indications and Dosage (Pediatric) in the drug label.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Cefotaxime sodium in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Cefotaxime sodium in pediatric patients.
Contraindications
- Cefotaxime is contraindicated in patients who have shown hypersensitivity to cefotaxime sodium, or the cephalosporin group of antibiotics.
Warnings
- BEFORE THERAPY WITH CEFOTAXIME IS INSTITUTED, CAREFUL INQUIRY SHOULD BE MADE TO DETERMINE WHETHER THE PATIENT HAS HAD PREVIOUS HYPERSENSITIVITY REACTIONS TO CEFOTAXIME SODIUM, CEPHALOSPORINS, PENICILLINS, OR OTHER DRUGS.
- THIS PRODUCT SHOULD BE GIVEN WITH CAUTION TO PATIENTS WITH TYPE I HYPERSENSITIVITY REACTIONS TO PENICILLIN.
- ANTIBIOTICS SHOULD BE ADMINISTERED WITH CAUTION TO ANY PATIENT WHO HAS DEMONSTRATED SOME FORM OF ALLERGY, PARTICULARLY TO DRUGS.
- IF AN ALLERGIC REACTION TO CEFOTAXIME OCCURS, DISCONTINUE TREATMENT WITH THE DRUG.
- SERIOUS HYPERSENSITIVITY REACTIONS MAY REQUIRE EPINEPHRINE AND OTHER EMERGENCY MEASURES.
- During post-marketing surveillance, a potentially life-threatening arrhythmia was reported in each of six patients who received a rapid (less than 60 seconds) bolus injection of cefotaxime through a central venous catheter.
- Clostridium difficile associated diarrhea (CDAD) has been reported with use of nearly all antibacterial agents, including cefotaxime, and may range in severity from mild diarrhea to fatal colitis.
- Treatment with antibacterial agents alters the normal flora of the colon leading to overgrowth of C. difficile.
- C. difficile produces toxins A and B which contribute to the development of CDA. Hypertoxin producing strains of C. difficile cause increased morbidity and mortality, as these infections can be refractory to antimicrobial therapy and may require colectomy.
- CDAD must be considered in all patients who present with diarrhea following antibiotic use.
- Careful medical history is necessary since CDAD has been reported to occur over two months after the administration of antibacterial agents.
- If CDAD is suspected or confirmed, ongoing antibiotic use not directed against C. difficile may need to be discontinued.
- Appropriate fluid and electrolyte management, protein supplementation, antibiotic treatment of C. difficile, and surgical evaluation should be instituted as clinically indicated.
Adverse Reactions
Clinical Trials Experience
Cefotaxime is generally well tolerated. The most common adverse reactions have been local reactions following IM or IV injection. Other adverse reactions have been encountered infrequently.
The most frequent adverse reactions (greater than 1%) are:
Local (4.3%)
- Injection site inflammation with IV administration
- Pain
- Induration
- Tenderness after IM injection
Hypersensitivity (2.4%)
Gastrointestinal (1.4%)
- Colitis
- Diarrhea
- Nausea, and vomiting (have been reported rarely.)
- Symptoms of pseudomembranous colitis can appear during or after antibiotic treatment.
Less frequent adverse reactions (less than 1%) are:
Hematologic System
- Neutropenia
- Transient leukopenia
- Some individuals have developed positive direct Coombs Tests during treatment with cefotaxime and other cephalosporin antibiotics.
Genitourinary System
Central Nervous System
Liver
Transient elevations in:
- AST
- ALT
- Serum LDH
- Serum alkaline phosphatase
Kidney
- As with some other cephalosporins, transient elevations of BUN have been occasionally observed with cefotaxime.
Postmarketing Experience
The following adverse reactions have been identified during post-approval use of cefotaxime. Because these reactions were reported voluntarily from a population of uncertain size, it is not possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Cardiovascular System
Potentially life-threatening arrhythmias following rapid (less than 60 seconds) bolus administration via central venous catheter have been observed.
Central Nervous System
Administration of high doses of beta-lactam antibiotics, including cefotaxime, particularly in patients with renal insufficiency may result in encephalopathy (e.g. impairment of consciousness, abnormal movements and convulsions).
Cutaneous
As with other cephalosporins, isolated cases of:
Hematologic System
Hypersensitivity
- Anaphylaxis (e.g., angioedema, bronchospasm, malaise possibly culminating in shock)
- Urticaria
Kidney
- Interstitial nephritis
- Transient elevations of creatinine.
Liver
- Hepatitis
- Jaundice
- Cholestasis
- Elevations of gamma GT and bilirubin.
Cephalosporin Class Labeling
In addition to the adverse reactions listed above which have been observed in patients treated with cefotaxime sodium, the following adverse reactions and altered laboratory tests have been reported for cephalosporin class antibiotics: allergic reactions, hepatic dysfunction including cholestasis, aplastic anemia, hemorrhage, and false-positive test for urinary glucose. Several cephalosporins have been implicated in triggering seizures, particularly in patients with renal impairment when the dosage was not reduced. If seizures associated with drug therapy occur, the drug should be discontinued. Anticonvulsant therapy can be given if clinically indicated.
Drug Interactions
- Increased nephrotoxicity has been reported following concomitant administration of cephalosporins and aminoglycoside antibiotics.
- Probenecid interferes with the renal tubular transfer of cefotaxime, decreasing the total clearance of cefotaxime by approximately 50% and increasing the plasma concentrations of cefotaxime. Administration of cefotaxime in excess of 6 grams/day should be avoided in patients receiving probenecid.
- A single intravenous dose and oral dose of probenecid (500 mg each) followed by two oral doses of probenecid 500 mg at approximately hourly intervals administered to three healthy male subjects receiving a continuous infusion of cefotaxime increased the steady-state plasma concentration of cefotaxime by approximately 80%. In another study, administration of oral probenecid 500 mg every 6 hours to six healthy male subjects with cefotaxime 1 gram infused over 5 minutes decreased the total clearance of cefotaxime by approximately 50%.
- Additionally, no disulfiram-like reactions were reported in a study conducted in 22 healthy volunteers administered cefotaxime and ethanol.
Use in Specific Populations
Pregnancy
- Reproduction studies have been performed in pregnant mice given cefotaxime intravenously at doses up to 1200 mg/kg/day (0.4 times the recommended human dose based on mg/m2) or in pregnant rats when administered intravenously at doses up to 1200 mg/kg/day (0.8 times the recommended human dose based on mg/m2).
- No evidence of embryotoxicity or teratogenicity was seen in these studies. *Although cefotaxime has been reported to cross the placental barrier and appear in cord blood, the effect on the human fetus is not known.
- There are no well-controlled studies in pregnant women. Because animal reproductive studies are not always predictive of human response, this drug should be used during pregnancy only if clearly needed.
Pregnancy Category (AUS): B1
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Cefotaxime sodium in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Cefotaxime sodium during labor and delivery.
Nursing Mothers
- Cefotaxime is excreted in human milk in low concentrations.
- Caution should be exercised when cefotaxime is administered to a nursing woman.
Pediatric Use
There is no FDA guidance on the use of Cefotaxime sodium in pediatric settings.
Geriatic Use
- Of the 1409 subjects in clinical studies of cefotaxime, 632 (45%) were 65 and over, while 258 (18%) were 75 and over.
- No overall differences in safety or effectiveness were observed between these subjects and younger subjects, and other reported clinical experience has not identified differences in responses between the elderly and younger patients, but greater sensitivity of some older individuals cannot be ruled out.
Gender
There is no FDA guidance on the use of Cefotaxime sodium with respect to specific gender populations.
Race
There is no FDA guidance on the use of Cefotaxime sodium with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Cefotaxime sodium in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Cefotaxime sodium in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Cefotaxime sodium in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Cefotaxime sodium in patients who are immunocompromised.
Administration and Monitoring
Administration
There is limited information regarding Cefotaxime sodium Administration in the drug label.
Monitoring
There is limited information regarding Cefotaxime sodium Monitoring in the drug label.
IV Compatibility
There is limited information regarding the compatibility of Cefotaxime sodium and IV administrations.
Overdosage
- The acute toxicity of cefotaxime was evaluated in neonatal and adult mice and rats.
- Significant mortality was seen at parenteral doses in excess of 6000 mg/kg/day in all groups.
- Common toxic signs in animals that died were a decrease in spontaneous activity, tonic and clonic convulsions, dyspnea, hypothermia, and cyanosis. *Cefotaxime sodium overdosage has occurred in patients.
- Most cases have shown no overt toxicity.
- The most frequent reactions were elevations of BUN and creatinine.
- There is a risk of reversible encephalopathy in cases of administration of high doses of beta-lactam antibiotics including cefotaxime.
- No specific antidote exists.
- Patients who receive an acute overdosage should be carefully observed and given supportive treatment.
Pharmacology
There is limited information regarding Cefotaxime sodium Pharmacology in the drug label.
Mechanism of Action
Cefotaxime sodium is a bactericidal agent that acts by inhibition of bacterial cell wall synthesis. Cefotaxime has activity in the presence of some of beta-lactamases, both penicillinases and cephalosporinases, of Gram-negative and Gram-positive bacteria.
Mechanism of Resistance
Resistance to cefotaxime is primarily through hydrolysis by beta-lactamase, alteration of penicillin-binding proteins (PBPs), and decreased permeability.
Structure
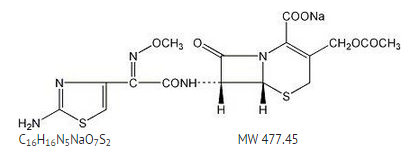
Sterile cefotaxime sodium is a semisynthetic, broad spectrum cephalosporin antibiotic for parenteral administration. It is the sodium salt of 7-[2-(2-amino-4-thiazolyl) glyoxylamido]-3(hydroxymethyl)-8-oxo-5-thia-1-azabicyclo [4.2.0] oct-2-ene-2-carboxylate 72 (Z)-(o-methyloxime), acetate (ester). Cefotaxime for Injection, USP contains approximately 50.5 mg (2.2 mEq) of sodium per gram of cefotaxime activity. Solutions of Cefotaxime for Injection, USP range from very pale yellow to light amber depending on the concentration and the diluent used. The pH of the injectable solutions usually ranges from 5.0 to 7.5.
Pharmacodynamics
There is limited information regarding Cefotaxime sodium Pharmacodynamics in the drug label.
Pharmacokinetics
There is limited information regarding Cefotaxime sodium Pharmacokinetics in the drug label.
Nonclinical Toxicology
There is limited information regarding Cefotaxime sodium Nonclinical Toxicology in the drug label.
Clinical Studies
There is limited information regarding Cefotaxime sodium Clinical Studies in the drug label.
How Supplied
There is limited information regarding Cefotaxime sodium How Supplied in the drug label.
Storage
There is limited information regarding Cefotaxime sodium Storage in the drug label.
Images
Drug Images
{{#ask: Page Name::Cefotaxime sodium |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
{{#ask: Label Page::Cefotaxime sodium |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
There is limited information regarding Cefotaxime sodium Patient Counseling Information in the drug label.
Precautions with Alcohol
No disulfiram-like reactions were reported in a study conducted in 22 healthy volunteers administered cefotaxime and ethanol.
Brand Names
- Claforan
Look-Alike Drug Names
There is limited information regarding Cefotaxime sodium Look-Alike Drug Names in the drug label.
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.