Cardiac amyloidosis pathophysiology
|
Cardiac amyloidosis Microchapters |
|
Diagnosis |
|---|
|
Treatment |
|
Case Studies |
|
Cardiac amyloidosis pathophysiology On the Web |
|
American Roentgen Ray Society Images of Cardiac amyloidosis pathophysiology |
|
Risk calculators and risk factors for Cardiac amyloidosis pathophysiology |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor: Aarti Narayan, M.B.B.S [2]; Raviteja Guddeti, M.B.B.S. [3]; Lakshmi Gopalakrishnan, M.B.B.S. [4]
Overview
The characteristic feature of cardiac amyloidosis is abnormal deposition of abnormally folded light chains of several serum proteins, making them insoluble and accumulate in various organs. This abnormal folding of proteins is most commonly a result of genetic mutations or excessive formation. Involvement of cardiac muscle can lead to heart failure, arrhythmias and advanced conduction disorder [1].
Pathophysiology
- Amyloidosis is characterized by the deposition and extracellular accumulation of fibrillary proteins, leading to the loss of normal tissue architecture.[2]
- Most frequent types are:
- Acquired monoclonal immunoglobulin light-chain amyloidosis
- Hereditary transthyretin (TTR)-related form
- Non-mutant TTR-related amyloidosis
Acquired monoclonal immunoglobulin light-chain amyloidosis (AL)
- The immunoglobulin lights chains of fibrillary deposits are produced by the clonal plasma cells in the bone marrow.
- Caused by greater than 100 mutations of TTR
- The protein is synthesized by the liver
Familial amyloidotic cardiomyopathy
- Defined as presence of TTR mutation that primarily affects the myocardium and without significant neuropathy.[7]
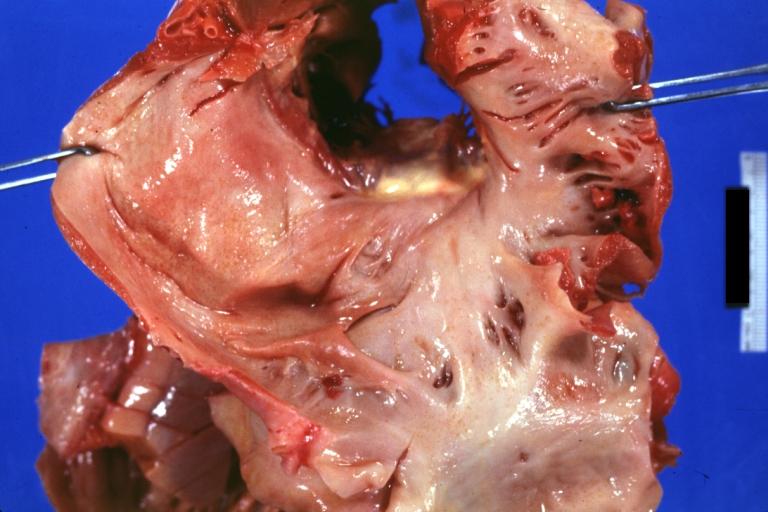
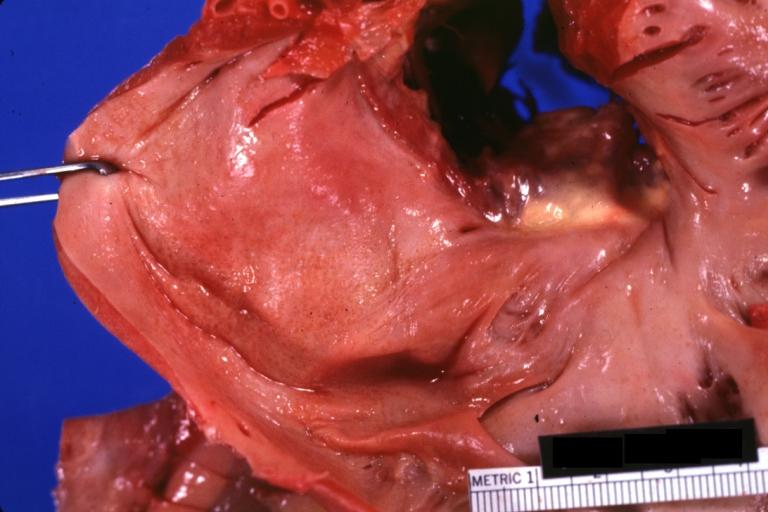
Gross Pathology
-
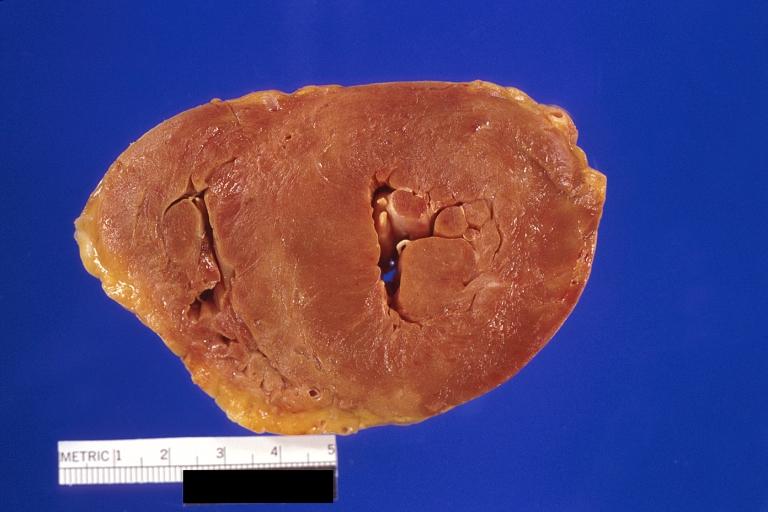
Amyloidosis Lesion In Left Atrium: Gross natural color view of a diagnostic lesion
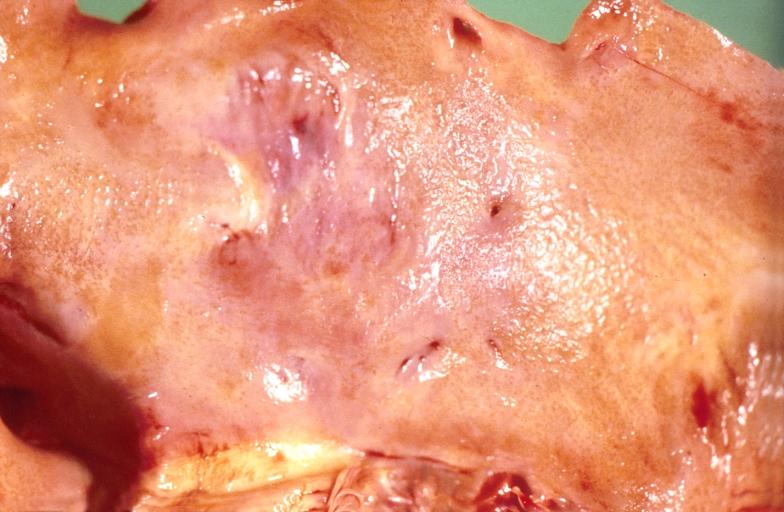
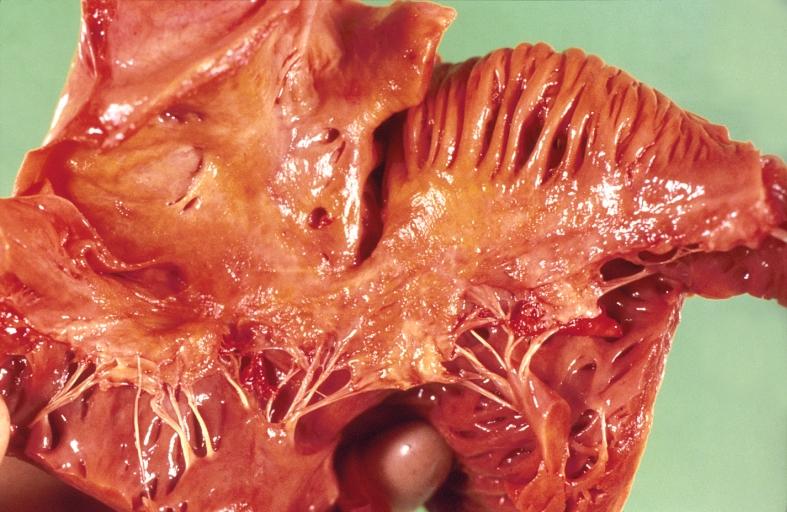
-
Amyloidosis Lesion In Left Atrium: Gross natural color close-up
Microscopic Pathology
-
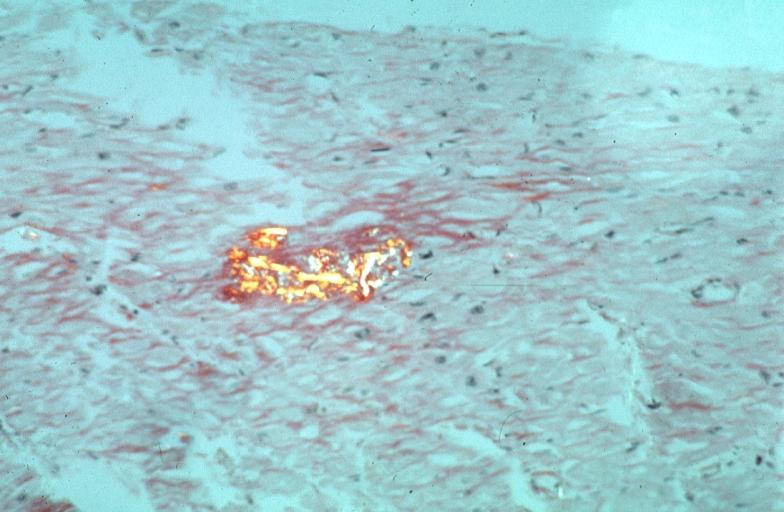
Heart: Perivascular amyloid, amyloidosis, congo red showing birefringence
-
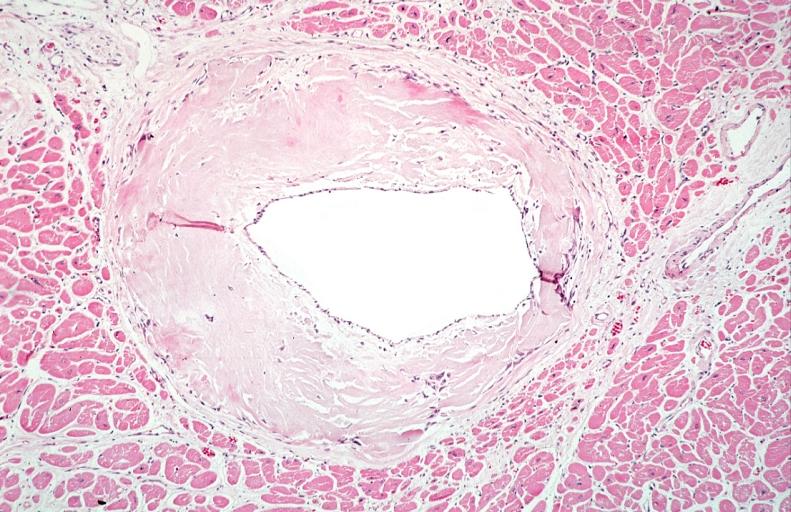
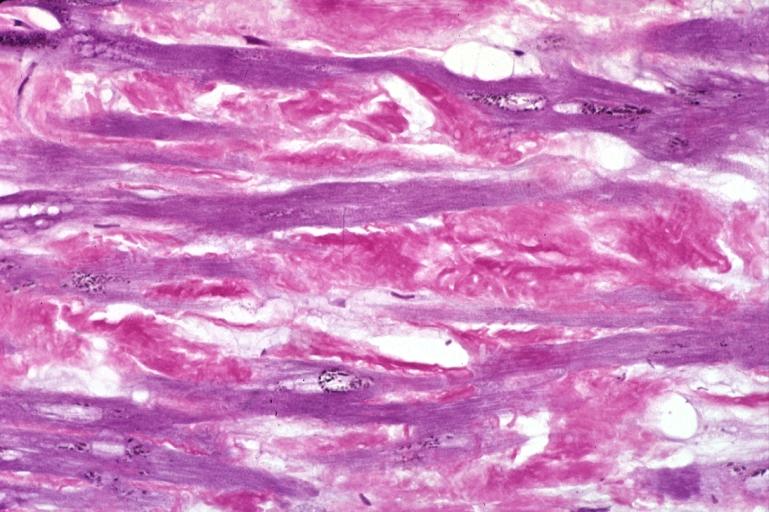
Heart: Perivascular amyloid, amyloidosis (Hematoxylin and eosin staining)
References
- ↑ Dharmarajan K, Maurer MS (2012). "Transthyretin cardiac amyloidoses in older North Americans". Journal of the American Geriatrics Society. 60 (4): 765–74. doi:10.1111/j.1532-5415.2011.03868.x. PMC 3325376. PMID 22329529. Unknown parameter
|month=ignored (help) - ↑ Merlini G, Bellotti V (2003). "Molecular mechanisms of amyloidosis". The New England Journal of Medicine. 349 (6): 583–96. doi:10.1056/NEJMra023144. PMID 12904524. Retrieved 2012-02-13. Unknown parameter
|month=ignored (help) - ↑ Falk RH, Comenzo RL, Skinner M (1997). "The systemic amyloidoses". The New England Journal of Medicine. 337 (13): 898–909. doi:10.1056/NEJM199709253371306. PMID 9302305. Retrieved 2012-02-13. Unknown parameter
|month=ignored (help) - ↑ Gertz MA, Lacy MQ, Dispenzieri A (1999). "Amyloidosis: recognition, confirmation, prognosis, and therapy". Mayo Clinic Proceedings. Mayo Clinic. 74 (5): 490–4. doi:10.4065/74.5.490. PMID 10319082. Retrieved 2012-02-13. Unknown parameter
|month=ignored (help) - ↑ Hodkinson HM, Pomerance A (1977). "The clinical significance of senile cardiac amyloidosis: a prospective clinico-pathological study". The Quarterly Journal of Medicine. 46 (183): 381–7. PMID 918253. Retrieved 2012-02-13. Unknown parameter
|month=ignored (help) - ↑ Lie JT, Hammond PI (1988). "Pathology of the senescent heart: anatomic observations on 237 autopsy studies of patients 90 to 105 years old". Mayo Clinic Proceedings. Mayo Clinic. 63 (6): 552–64. PMID 3374172. Unknown parameter
|month=ignored (help);|access-date=requires|url=(help) - ↑ Buxbaum JN, Tagoe CE (2000). "The genetics of the amyloidoses". Annual Review of Medicine. 51: 543–69. doi:10.1146/annurev.med.51.1.543. PMID 10774481. Retrieved 2012-02-13.